AP Physics 2- 9.6 Entropy and the Second Law of Thermodynamics- Study Notes- New Syllabus
AP Physics 2- 9.6 Entropy and the Second Law of Thermodynamics – Study Notes
AP Physics 2- 9.6 Entropy and the Second Law of Thermodynamics – Study Notes – per latest Syllabus.
Key Concepts:
- Entropy and Second Law of Thermodynamics
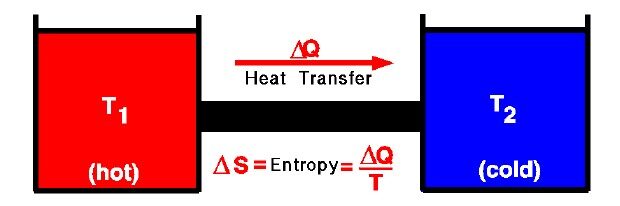
Entropy: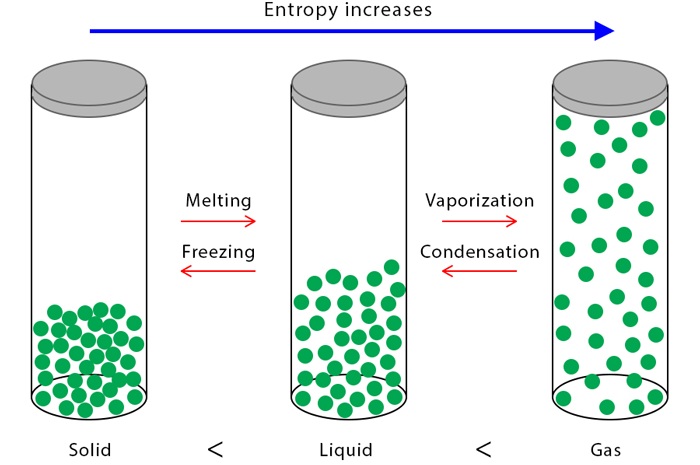
Entropy measures the tendency of energy to spread out or the unavailability of energy to do work.
- Localized energy will naturally disperse over time.
- Entropy is a state function: it depends only on the current state or configuration of the system, not on the path taken.
- Maximum entropy occurs when a system reaches thermodynamic equilibrium.
- The change in a system’s entropy depends on interactions with its surroundings.
Mathematical Expression:
\( \Delta S = \displaystyle \int \dfrac{dQ_{rev}}{T} \)
- \( \Delta S \): Change in entropy
- \( dQ_{rev} \): Infinitesimal heat absorbed reversibly
- \( T \): Absolute temperature at which heat is transferred
Essential conclusions:
- Entropy is a measure of energy dispersal and disorder.
- Isolated systems always move toward maximum entropy (thermodynamic equilibrium).
- Reversible processes maintain total entropy, irreversible processes increase it.
- Entropy changes in a system are influenced by energy exchanges with surroundings.

Second Law of Thermodynamics:
The total entropy of an isolated system can never decrease. It remains constant only when all processes are reversible.
- Isolated systems spontaneously evolve toward thermodynamic equilibrium, the state of maximum entropy.
- In a closed system, entropy can decrease because energy can be exchanged with the surroundings.
Example :
One mole of an ideal gas expands reversibly and isothermally at 300 K from 5 L to 10 L. Find the entropy change.
▶️ Answer/Explanation
Entropy change for isothermal expansion: \( \Delta S = n R \ln \dfrac{V_f}{V_i} \)
\( \Delta S = 1 \times 8.314 \ln \dfrac{10}{5} = 8.314 \ln 2 \approx 5.76 \, J/K \)
Answer: Entropy change ≈ 5.76 J/K.
Example :
Heat of 400 J is removed reversibly from a system at 500 K. Find the change in entropy.
▶️ Answer/Explanation
\( \Delta S = \dfrac{Q_{rev}}{T} = \dfrac{-400}{500} = -0.8 \, J/K \)
Answer: Change in entropy = -0.8 J/K.