AP Physics C E&M- 8.1 Electrostatics Charge and Electric Force - Study Notes- New Syllabus
AP Physics C E&M- 8.1 Electrostatics Charge and Electric Force – Study Notes
AP Physics C E&M- 8.1 Electrostatics Charge and Electric Force – Study Notes – per latest Syllabus.
Key Concepts:
- Electric Charge
- Coulomb’s Law
- Electric and Gravitational Forces Between Charged Objects with Mass
- Electric Permittivity of a Material or Medium
Electric Charge
Electric charge is a fundamental property of matter that gives rise to electric forces and interactions. Objects can be charged either positively or negatively, depending on the imbalance of protons and electrons.
Basic Properties of Charge:![]()
Two Types: Positive (+) and Negative (−). Like charges repel, unlike charges attract.
Quantization of Charge: Electric charge exists in discrete packets, integral multiples of the elementary charge:
$ \mathrm{q = n e}, \quad n = \pm 1, \pm 2, \dots $ where \(\mathrm{e = 1.6 \times 10^{-19} \, C}\).
Conservation of Charge: The total charge in an isolated system remains constant during any physical or chemical process.
Conductors and Insulators:
- Conductors: Materials in which charges move freely (e.g., metals).
- Insulators: Materials in which charges cannot move freely (e.g., rubber, glass).
- Semi-conductors: Materials whose conductivity lies between conductors and insulators, and can be controlled (e.g., silicon).
Example :
If a body has a net charge of \(\mathrm{-4.8 \times 10^{-19} \, C}\), determine how many excess electrons it possesses.
▶️ Answer/Explanation
Step 1: Use quantization of charge: \(\mathrm{q = n e}\)
Step 2: \(\mathrm{n = \frac{q}{e} = \frac{-4.8 \times 10^{-19}}{1.6 \times 10^{-19}} = -3}\)
Step 3: The negative sign means excess electrons. Number of excess electrons = 3.
Final Answer: The body has 3 excess electrons.
Coulomb’s Law
Coulomb’s law describes the electrostatic force between two point charges. The force depends on the product of the charges and the inverse square of the distance between them.
Mathematical Form: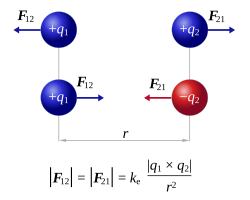
$ \mathrm{F = k_e \frac{|q_1 q_2|}{r^2}} $
- \(\mathrm{F}\) = magnitude of the electrostatic force
- \(\mathrm{q_1, q_2}\) = interacting charges
- \(\mathrm{r}\) = distance between charges
- \(\mathrm{k_e = 8.99 \times 10^9 \, N \, m^2/C^2}\) (Coulomb’s constant)
Direction of the Electrostatic Force:
The force acts along the straight line joining the two charges.
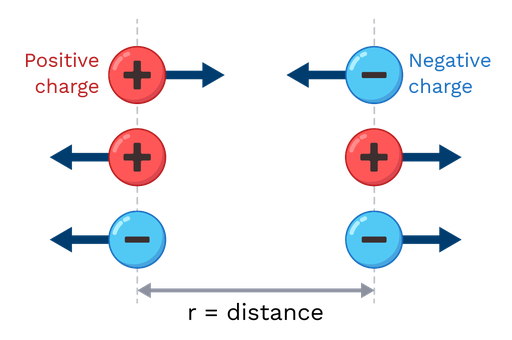
- If charges have the same sign (\(+,+\) or \(−,−\)): the force is repulsive, directed away from each other.
- If charges have opposite signs (\(+\) and \(−\)): the force is attractive, directed toward each other.
In vector form:
$ \mathrm{\vec{F}_{12} = k_e \frac{q_1 q_2}{r^2} \, \hat{r}_{12}} $ where \(\mathrm{\hat{r}_{12}}\) is the unit vector from charge 1 to charge 2.
Superposition Principle:
If more than two charges are present, the net force on a given charge is the vector sum of forces due to all other charges.
Example :
Two charges, \(\mathrm{q_1 = +2 \, \mu C}\) and \(\mathrm{q_2 = +3 \, \mu C}\), are placed \(\mathrm{0.4 \, m}\) apart in vacuum. Find the magnitude and direction of the force on \(\mathrm{q_2}\).
▶️ Answer/Explanation
Step 1: Use Coulomb’s law: \(\mathrm{F = k_e \frac{|q_1 q_2|}{r^2}}\)
Step 2: Substitute values: \(\mathrm{F = (8.99 \times 10^9) \frac{(2 \times 10^{-6})(3 \times 10^{-6})}{(0.4)^2}}\)
Step 3: \(\mathrm{F = (8.99 \times 10^9) \frac{6 \times 10^{-12}}{0.16} \approx 0.34 \, N}\)
Step 4: Since both charges are positive, the force is repulsive. The force on \(\mathrm{q_2}\) is directed away from \(\mathrm{q_1}\).
Final Answer: \(\mathrm{F \approx 0.34 \, N}\), directed away from \(\mathrm{q_1}\).
Electric and Gravitational Forces Between Charged Objects with Mass
Objects that have both electric charge and mass interact through two fundamental forces: the electrostatic force (due to charge) and the gravitational force (due to mass).
Electrostatic Force:![]()
$ \mathrm{F_e = k_e \frac{|q_1 q_2|}{r^2}} $
- \(\mathrm{F_e}\) = magnitude of the electric force
- \(\mathrm{q_1, q_2}\) = charges of the objects
- \(\mathrm{r}\) = distance between charges
- \(\mathrm{k_e = 8.99 \times 10^9 \, N \, m^2 / C^2}\)
- Direction: Attractive if charges are opposite; repulsive if charges are the same.
Gravitational Force:![]()
$ \mathrm{F_g = G \frac{m_1 m_2}{r^2}} $
- \(\mathrm{F_g}\) = magnitude of the gravitational force
- \(\mathrm{m_1, m_2}\) = masses of the objects
- \(\mathrm{r}\) = distance between objects
- \(\mathrm{G = 6.67 \times 10^{-11} \, N \, m^2 / kg^2}\)
- Direction: Always attractive.
Comparison of Forces:
- Both vary as the inverse square of distance (\(\mathrm{1/r^2}\)).
- Electric forces can be either attractive or repulsive, while gravitational forces are always attractive.
- The electric force is vastly stronger than the gravitational force for elementary particles.
Example :
Compare the magnitudes of electric and gravitational forces between two protons separated by \(\mathrm{1.0 \times 10^{-10} \, m}\).
▶️ Answer/Explanation
Step 1: Electric force: \(\mathrm{F_e = k_e \frac{q^2}{r^2}}\) \(\mathrm{= (8.99 \times 10^9) \frac{(1.6 \times 10^{-19})^2}{(1.0 \times 10^{-10})^2}}\)
\(\mathrm{F_e \approx 2.3 \times 10^{-8} \, N}\)
Step 2: Gravitational force: \(\mathrm{F_g = G \frac{m_p^2}{r^2}}\), where \(\mathrm{m_p = 1.67 \times 10^{-27} \, kg}\) \(\mathrm{= (6.67 \times 10^{-11}) \frac{(1.67 \times 10^{-27})^2}{(1.0 \times 10^{-10})^2}}\)
\(\mathrm{F_g \approx 1.9 \times 10^{-44} \, N}\)
Step 3: Ratio: \(\mathrm{\frac{F_e}{F_g} \approx 10^{36}}\)
Final Answer: The electric force between two protons is about \(\mathrm{10^{36}}\) times stronger than the gravitational force.
Electric Permittivity of a Material or Medium
Electric permittivity is a property of a material that determines how easily electric field lines pass through it. It describes how a medium affects the strength of the electric force between charges and the behavior of electric fields inside the medium.
Absolute Permittivity:![]()
$ \mathrm{\varepsilon = \varepsilon_r \varepsilon_0} $
- \(\mathrm{\varepsilon}\) = absolute permittivity of the medium
- \(\mathrm{\varepsilon_r}\) = relative permittivity (also called dielectric constant)
- \(\mathrm{\varepsilon_0 = 8.85 \times 10^{-12} \, C^2/(N \, m^2)}\) = permittivity of free space
Relative Permittivity (Dielectric Constant):
It is the ratio of permittivity of the medium to that of free space:
$ \mathrm{\varepsilon_r = \frac{\varepsilon}{\varepsilon_0}} $
- \(\mathrm{\varepsilon_r > 1}\) for all materials other than vacuum.
- It indicates how much the medium reduces the effective electric field compared to vacuum.
Effect on Coulomb’s Law:
In a medium, the electrostatic force between two point charges is reduced compared to vacuum:
$ \mathrm{F = \frac{1}{4 \pi \varepsilon} \frac{|q_1 q_2|}{r^2}} $
$ \mathrm{= \frac{1}{4 \pi \varepsilon_0 \varepsilon_r} \frac{|q_1 q_2|}{r^2}} $
Key Points:
- A higher permittivity means the medium can store more electric energy per unit volume when placed in an electric field.
- Materials with high \(\mathrm{\varepsilon_r}\) (like water, \(\mathrm{\varepsilon_r \approx 80}\)) strongly reduce the electric field.
- Vacuum has the lowest possible permittivity, \(\mathrm{\varepsilon_r = 1}\).
Example :
Two charges of \(\mathrm{+1 \, \mu C}\) each are separated by \(\mathrm{0.1 \, m}\). Calculate the force between them in vacuum and in water (\(\mathrm{\varepsilon_r = 80}\)).
▶️ Answer/Explanation
Step 1: Force in vacuum: \(\mathrm{F_0 = \frac{1}{4 \pi \varepsilon_0} \frac{q^2}{r^2}}\)
\(\mathrm{= (9 \times 10^9) \frac{(1 \times 10^{-6})^2}{(0.1)^2} = 0.9 \, N}\)
Step 2: Force in water: \(\mathrm{F = \frac{F_0}{\varepsilon_r} = \frac{0.9}{80} \approx 0.011 \, N}\)
Final Answer: Force is \(\mathrm{0.9 \, N}\) in vacuum and only \(\mathrm{0.011 \, N}\) in water.
