1.2C Particle Attractions and Phase Changes- Pre AP Chemistry Study Notes - New Syllabus.
1.2C Particle Attractions and Phase Changes- Pre AP Chemistry Study Notes
1.2C Particle Attractions and Phase Changes- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
1.2.C.1 Explain the relationship between changes in states of matter and the attractions among particles.
1.2.C.2 Create and/or interpret models representing phase changes.
Key Concepts:
- 1.2.C Substances with stronger attractions among particles generally have higher melting and boiling points than substances with weaker attractions among particles.
1.2.C.1 — States of Matter and Attractions Among Particles
Changes in the state of matter (solid, liquid, gas) are closely related to the strength of attractions among particles. These attractions determine how easily particles can move apart when thermal energy is added.
As a result, substances with stronger particle attractions generally have higher melting points and boiling points than substances with weaker attractions.
Attractions Among Particles
Particles attract each other due to intermolecular (or interparticle) forces. The strength of these attractions controls how tightly particles are held together.
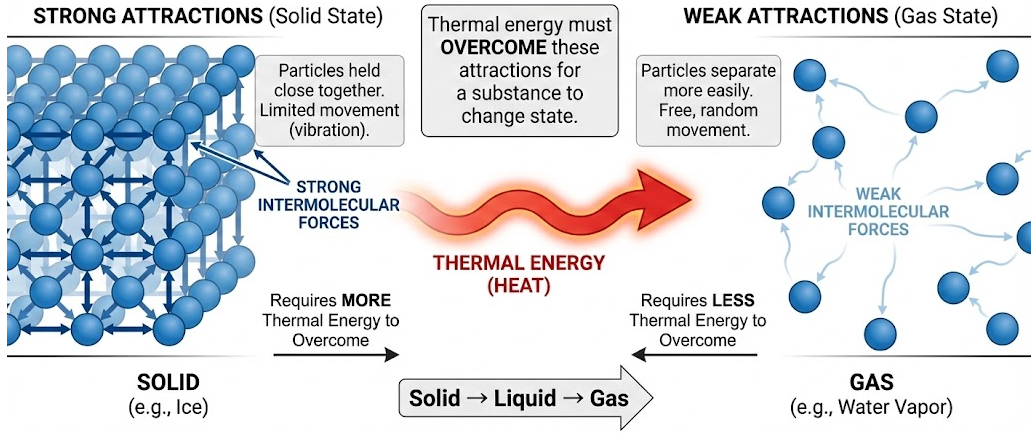
- Strong attractions hold particles close together
- Weak attractions allow particles to separate more easily
Thermal energy must overcome these attractions for a substance to change state.
States of Matter and Particle Attractions
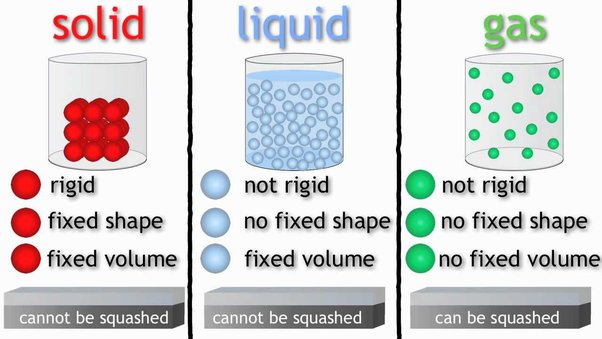
Solids
| Liquids
| Gases
|
Melting and Boiling: Role of Particle Attractions
During a phase change, thermal energy is used to overcome attractions between particles, not to increase temperature.
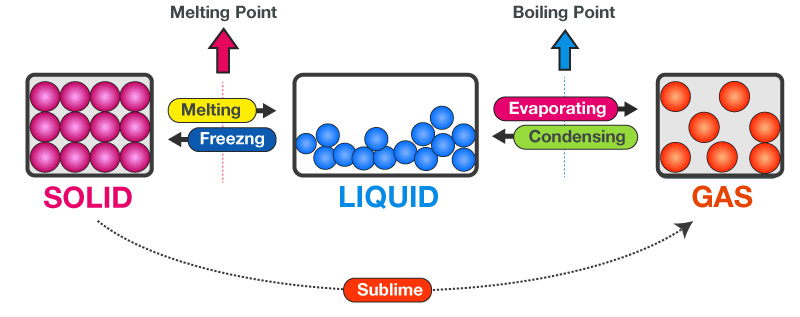
- Melting: particles partially overcome attractions and move more freely
- Boiling: particles completely overcome attractions and separate into a gas
Stronger attractions require more energy to overcome, leading to higher melting and boiling points.
Attraction Strength and Phase-Change Temperatures
| Strength of Particle Attractions | Melting Point | Boiling Point |
|---|---|---|
| Strong | High | High |
| Moderate | Moderate | Moderate |
| Weak | Low | Low |
Evaluating Particulate Models
A correct particulate model explaining phase changes should:
- Show particles becoming more separated as attractions are overcome
- Keep particle type and size constant
- Relate increased separation to added thermal energy
Models that show particles breaking apart or changing type are incorrect for phase changes.
Example
Explain why a substance with strong attractions among particles has a high melting point.
▶️ Answer / Explanation
Strong attractions hold particles tightly together. A large amount of thermal energy is needed to overcome these attractions, so the substance only melts at a high temperature.
Example
Two pure substances are heated at the same rate. Substance A boils at a much higher temperature than substance B. Using particle attractions, explain this difference.
▶️ Answer / Explanation
Substance A has stronger attractions among its particles than substance B. More thermal energy is required to fully overcome these attractions, so particles in substance A only separate into a gas at a higher temperature. Substance B has weaker attractions, so it boils at a lower temperature.
1.2.C.2 — Models Representing Phase Changes
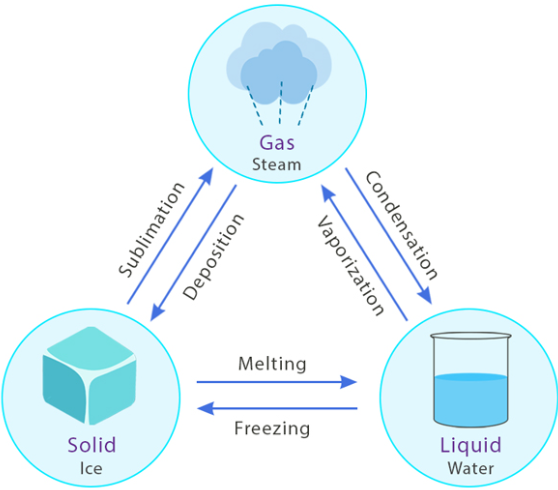
Phase changes (melting, freezing, vaporization, condensation, sublimation, deposition) can be explained using particle (particulate) models and graphical models. These models show how particles respond when thermal energy is transferred.
What Changes During a Phase Change?
During a phase change:
- The state of matter changes
- The temperature remains constant
- Thermal energy is used to overcome or form attractions among particles
Temperature does not increase during a phase change because the added energy does not increase particle speed; it changes particle arrangement.
Particulate Models of Phase Changes
Particulate models represent phase changes by showing how particle spacing, motion, and attractions change.
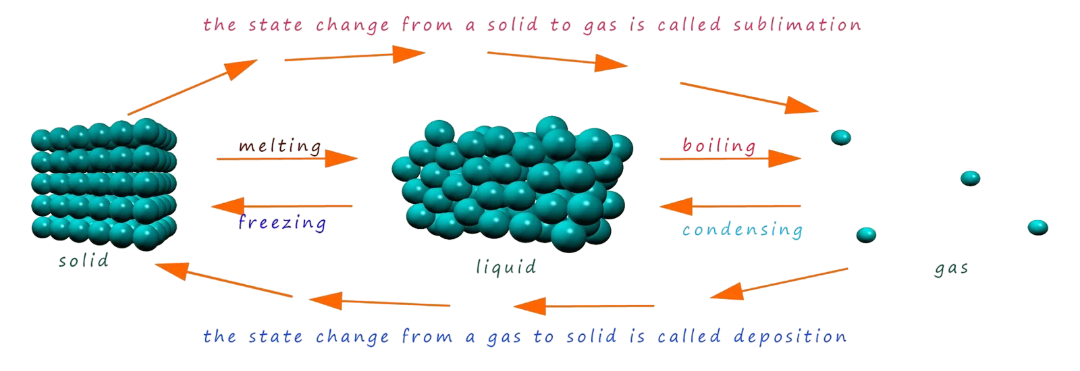
Melting (Solid → Liquid)
![]()
- Particles gain energy
- Attractions are partially overcome
- Particles move from fixed positions to sliding motion
Boiling / Vaporization (Liquid → Gas)
![]()
- Particles gain enough energy to fully overcome attractions
- Particles become far apart and move freely
Freezing (Liquid → Solid)
![]()
- Energy is released
- Attractions pull particles into fixed positions
Condensation (Gas → Liquid)
![]()
- Particles lose energy
- Attractions pull particles closer together
Graphical Models: Heating and Cooling Curves
A heating curve is a graph of temperature versus thermal energy added. It visually represents phase changes.
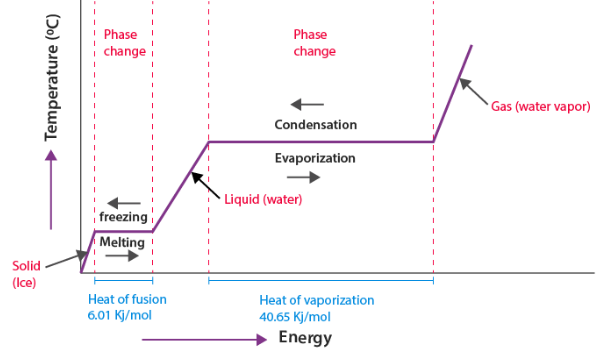
- Sloped sections → temperature changing within a single phase
- Flat sections → phase changes occurring
During flat sections, energy goes into breaking attractions rather than raising temperature.
Interpreting Phase-Change Models
| Model Feature | What It Represents |
|---|---|
| Increasing particle spacing | Weaker attractions / phase change to gas |
| Constant temperature plateau | Energy used for phase change |
| Closer particle arrangement | Stronger attractions / solid or liquid |
Evaluating the Accuracy of Models
A correct phase-change model must:
- Keep particle identity and size constant
- Show changes in spacing and arrangement
- Show constant temperature during the phase change
Models that show particles breaking apart or changing type are incorrect for phase changes.
Example
A heating curve shows a flat section where temperature remains constant as energy is added. Identify what is occurring and explain using particle behavior.
▶️ Answer / Explanation
A phase change is occurring.
The added thermal energy is used to overcome attractions among particles rather than increase their average kinetic energy, so the temperature stays constant.
Example
Two particulate models represent the same substance. Model A shows particles closely packed and vibrating. Model B shows particles farther apart with sliding motion. Identify the phase change and explain the energy transfer involved.
▶️ Answer / Explanation
The phase change is melting (solid to liquid).
Thermal energy is absorbed by the substance, allowing particles to partially overcome attractive forces and move more freely while remaining close together.
