2.1A Pure Substances- Pre AP Chemistry Study Notes - New Syllabus.
2.1A Pure Substances- Pre AP Chemistry Study Notes
2.1A Pure Substances- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
2.1.A.1 Distinguish between atoms, molecules, and compounds at the particle level.
2.1.A.2 Create and/or evaluate models of pure substances.
Key Concepts:
- 2.1.A A pure substance always has the same composition. Pure substances include elements, molecules, and compounds.
a. An element is composed of only one type of atom.
b. A molecule is a particle composed of more than one atom.
c. A compound is composed of two or more elements and has properties distinct from those of its component atoms.
2.1.A.1 — Atoms, Molecules, and Compounds at the Particle Level
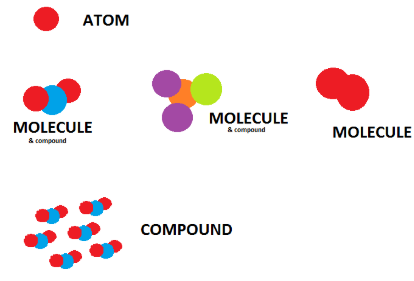
At the particle level, matter can be described in terms of atoms, molecules, and compounds. Distinguishing between these particles helps explain the composition and behavior of substances.
Atoms
An atom is the smallest particle of an element that retains the properties of that element.
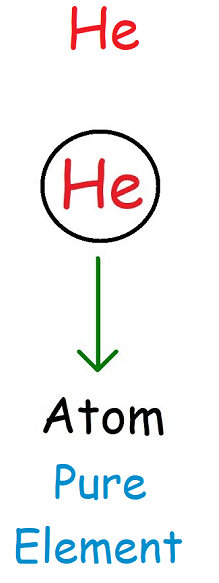
- Consists of one type of element
- Cannot be broken down by chemical means
- May exist alone (e.g., noble gases)
In particle models, atoms are shown as single, unbonded spheres.
Molecules
A molecule is a particle made of two or more atoms chemically bonded together.
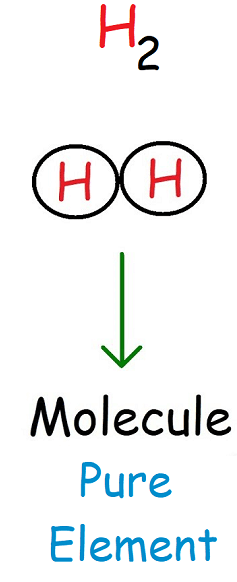
- Atoms in a molecule can be the same element or different elements
- The smallest independent unit of many substances
In particle models, molecules are shown as groups of bonded spheres.
Compounds
A compound is a substance made of two or more different elements chemically bonded in a fixed ratio.
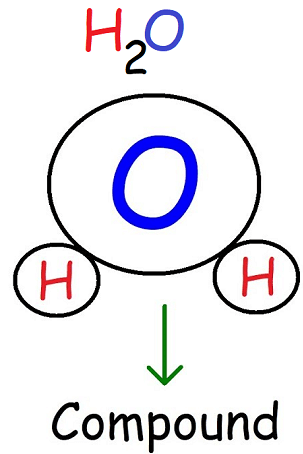
- Always contains more than one type of atom
- Has properties different from the individual elements
- Can be broken down only by chemical reactions
At the particle level, compounds can exist as:
- Molecular compounds (discrete molecules, e.g., water)
- Ionic compounds (repeating ion patterns, not single molecules)
Comparing Atoms, Molecules, and Compounds
| Particle Type | Composition | Particle-Level Description |
|---|---|---|
| Atom | One element | Single, unbonded particle |
| Molecule | Two or more atoms | Bonded group of atoms |
| Compound | Two or more different elements | Bonded atoms in fixed ratios |
Key Distinctions at the Particle Level
- All compounds contain molecules or repeating ion units, but not all molecules are compounds
- Molecules made of only one element are not compounds
- Atoms are the building blocks of both molecules and compounds
Example
A particle model shows two identical atoms bonded together. Identify the particle and justify your answer.
▶️ Answer / Explanation
The particle is a molecule.
It consists of two atoms that are chemically bonded. Because both atoms are the same element, it is not a compound.
Example
A sample contains particles, each made of one large sphere bonded to two smaller, differently colored spheres. Distinguish whether the particles represent atoms, molecules, or compounds, and justify using particle-level reasoning.
▶️ Answer / Explanation
The particles represent a compound.
Each particle consists of atoms of different elements bonded together in a fixed arrangement, which meets the definition of a compound at the particle level.
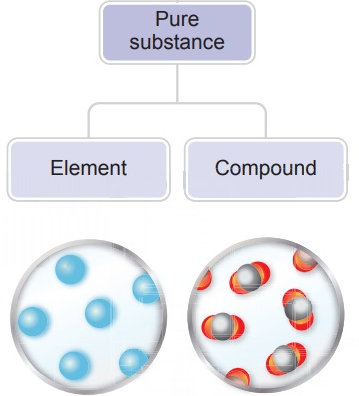
2.1.A.2 — Models of Pure Substances
Pure substances are made of only one type of particle. Particle models are used to represent and evaluate whether a sample is a pure substance by examining the uniformity and identity of its particles.
What Is a Pure Substance?
A pure substance has: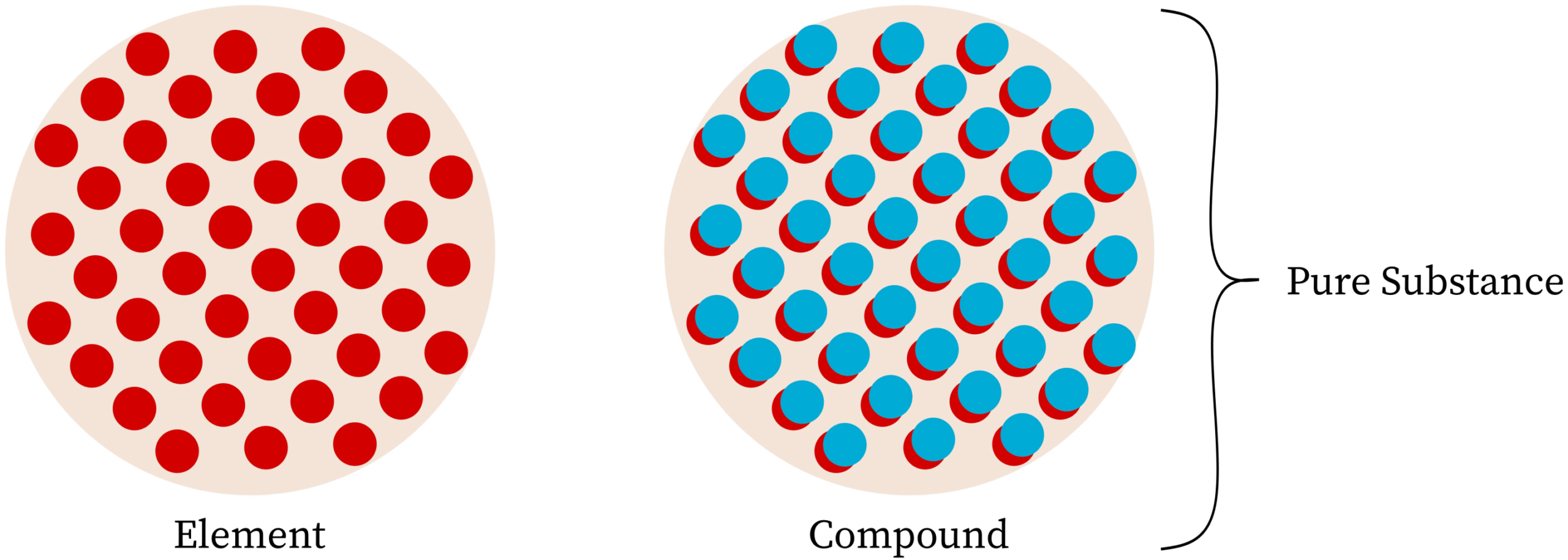
- Only one type of particle throughout the sample
- A fixed composition
- Uniform properties at all locations
Pure substances can be:
- Elements (single type of atom)
- Compounds (particles made of different atoms bonded in fixed ratios)
Particle Models of Pure Substances
In a correct particle model of a pure substance:
- All particles are identical
- No other particle types are present
- Particles may be atoms, molecules, or repeating ion units
Uniformity is the key feature that distinguishes pure substances from mixtures.
Models of Pure Elements
A pure element consists of only one type of atom.
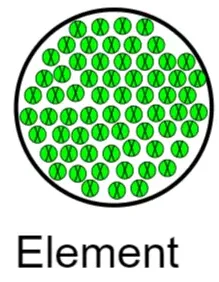
- Particles may appear as single atoms
- Or as molecules made of the same element (e.g., diatomic molecules)
In models, all particles look the same in size and color.
Models of Pure Compounds
A pure compound contains particles made of different elements bonded together in a fixed ratio.
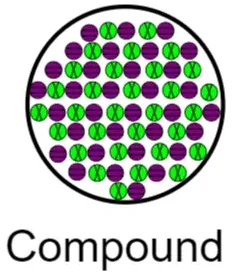
- Every particle has the same combination of atoms
- Different colors or sizes represent different elements
- The ratio of atoms is constant
If all compound particles are identical, the substance is pure.
Distinguishing Pure Substances from Mixtures Using Models
| Model Feature | Pure Substance | Mixture |
|---|---|---|
| Types of particles | One type | More than one type |
| Particle uniformity | All identical | Not identical |
| Composition | Fixed | Variable |
Evaluating the Accuracy of Pure-Substance Models
A correct model of a pure substance must:
- Show only one type of particle
- Keep particle size consistent for the same element
- Avoid mixing atoms or molecules of different types
If a model shows more than one particle type, it represents a mixture—not a pure substance.
Example
A particle model shows many identical pairs of bonded atoms, all the same color. Determine whether the sample represents a pure substance and justify your answer.
▶️ Answer / Explanation
The sample represents a pure substance.
All particles are identical molecules made of the same element, so only one type of particle is present.
Example
A diagram shows particles where each consists of one large sphere bonded to two smaller spheres. All particles are identical. Evaluate whether the diagram represents a pure substance and explain your reasoning.
▶️ Answer / Explanation
The diagram represents a pure substance.
Although each particle contains different elements, every particle has the same composition and structure, which indicates a pure compound rather than a mixture.
