2.2B Intermolecular Forces and Physical Properties- Pre AP Chemistry Study Notes - New Syllabus.
2.2B Intermolecular Forces and Physical Properties- Pre AP Chemistry Study Notes
2.2B Intermolecular Forces and Physical Properties- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
2.2.B.1 Create and/or evaluate a claim that uses relative strength of intermolecular forces to explain trends in the physical properties of substances.
Key Concepts:
- 2.2.B Intermolecular forces can be used to explain trends in physical properties of substances including boiling point, melting point, surface tension, volatility, and solubility.
2.2.B.1 — Intermolecular Forces and Trends in Physical Properties
The relative strength of intermolecular forces (IMF) can be used to create and evaluate claims explaining trends in the physical properties of substances. Because IMF arise from electrostatic attractions, stronger IMF require more energy to overcome.
As intermolecular force strength increases, predictable trends appear in boiling point, melting point, surface tension, volatility, and solubility.
Principle
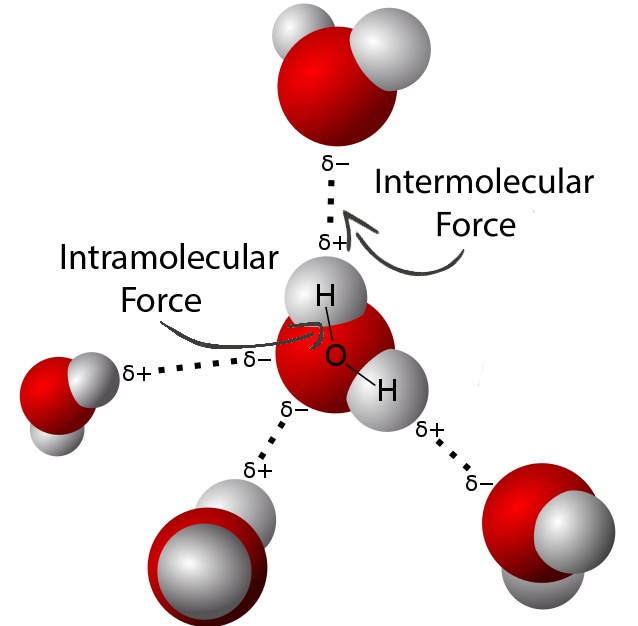
Stronger intermolecular forces → particles are held together more tightly → more energy is required to separate them.
Boiling Point
Boiling occurs when particles gain enough energy to overcome intermolecular forces and enter the gas phase.
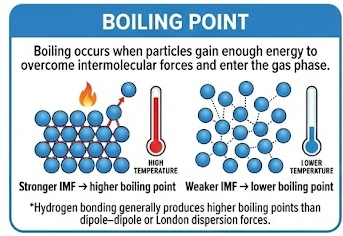
- Stronger IMF → higher boiling point
- Weaker IMF → lower boiling point
Hydrogen bonding generally produces higher boiling points than dipole–dipole or London dispersion forces.
Melting Point
Melting requires particles to partially overcome intermolecular attractions.
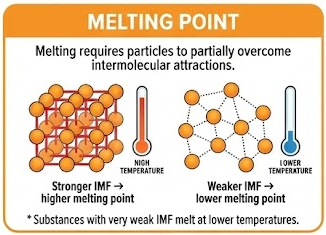
- Stronger IMF → higher melting point
- Weaker IMF → lower melting point
Substances with very weak IMF melt at lower temperatures.
Surface Tension
Surface tension is caused by strong attractive forces between particles at a liquid’s surface.
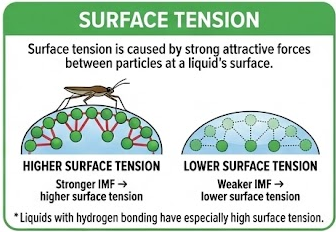
- Stronger IMF → higher surface tension
- Weaker IMF → lower surface tension
Liquids with hydrogen bonding have especially high surface tension.
Volatility
Volatility describes how easily particles escape from a liquid into the gas phase.
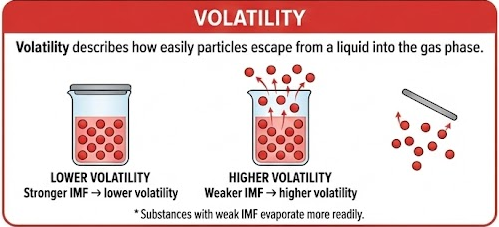
- Stronger IMF → lower volatility
- Weaker IMF → higher volatility
Substances with weak IMF evaporate more readily.
Solubility
Solubility depends on whether intermolecular forces between solute and solvent are strong enough to replace those within each substance.
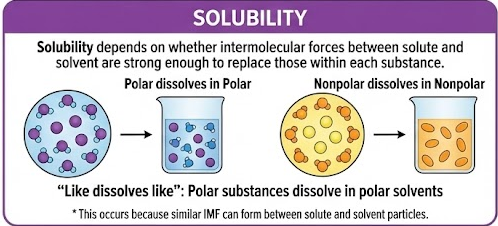
- “Like dissolves like”
- Polar substances dissolve in polar solvents
- Nonpolar substances dissolve in nonpolar solvents
This occurs because similar IMF can form between solute and solvent particles.
Summary of IMF Strength and Physical Properties
| Intermolecular Force Strength | Effect on Physical Properties |
|---|---|
| Stronger IMF | Higher boiling/melting point, higher surface tension, lower volatility |
| Weaker IMF | Lower boiling/melting point, lower surface tension, higher volatility |
Evaluating Claims About Trends
A correct claim must:
- Identify the relevant intermolecular forces
- Compare their relative strengths
- Link force strength to the observed physical property trend
Claims based only on molar mass or size without discussing IMF are incomplete.
Example
Create a claim explaining why water has a higher boiling point than methane.
▶️ Answer / Explanation
Water has a higher boiling point than methane because it experiences stronger intermolecular forces.
Water molecules form hydrogen bonds, while methane only experiences London dispersion forces. Stronger intermolecular attractions in water require more energy to overcome during boiling.
Example
Pentane (C₅H₁₂) and hexane (C₆H₁₄) are both nonpolar liquids. Create and evaluate a claim explaining the difference in their boiling points.
▶️ Answer / Explanation
Hexane has a higher boiling point than pentane.
Hexane has a larger molar mass and more electrons, resulting in stronger London dispersion forces. Stronger intermolecular attractions require more energy to overcome, leading to a higher boiling point.
