2.2F Molecular Polarity- Pre AP Chemistry Study Notes - New Syllabus.
2.2F Molecular Polarity- Pre AP Chemistry Study Notes
2.2F Molecular Polarity- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
2.2.F.1 Determine the polarity of a molecule from its molecular geometry and electron distribution.
Key Concepts:
- 2.2.F Molecules with asymmetric distributions of electrons are polar.
2.2.F.1 — Molecular Polarity from Geometry and Electron Distribution
Molecular polarity depends on how electrons are distributed within a molecule. Even when individual bonds are polar, the overall molecule may be polar or nonpolar depending on its molecular geometry.
According to this principle, molecules with asymmetric distributions of electrons are polar. To determine polarity, both bond polarity and molecular shape must be considered.
Step 1: Bond Polarity (Electron Distribution)
A bond is polar when there is a difference in electronegativity between bonded atoms, causing electrons to be shared unequally.

- Unequal sharing → partial charges (\( \mathrm{\delta^+} \), \( \mathrm{\delta^-} \))
- Equal sharing → nonpolar bond
Polar bonds create bond dipoles, which point toward the more electronegative atom.
Step 2: Molecular Geometry (Shape)
Molecular geometry determines whether bond dipoles cancel or reinforce each other. This geometry is predicted using VSEPR theory.
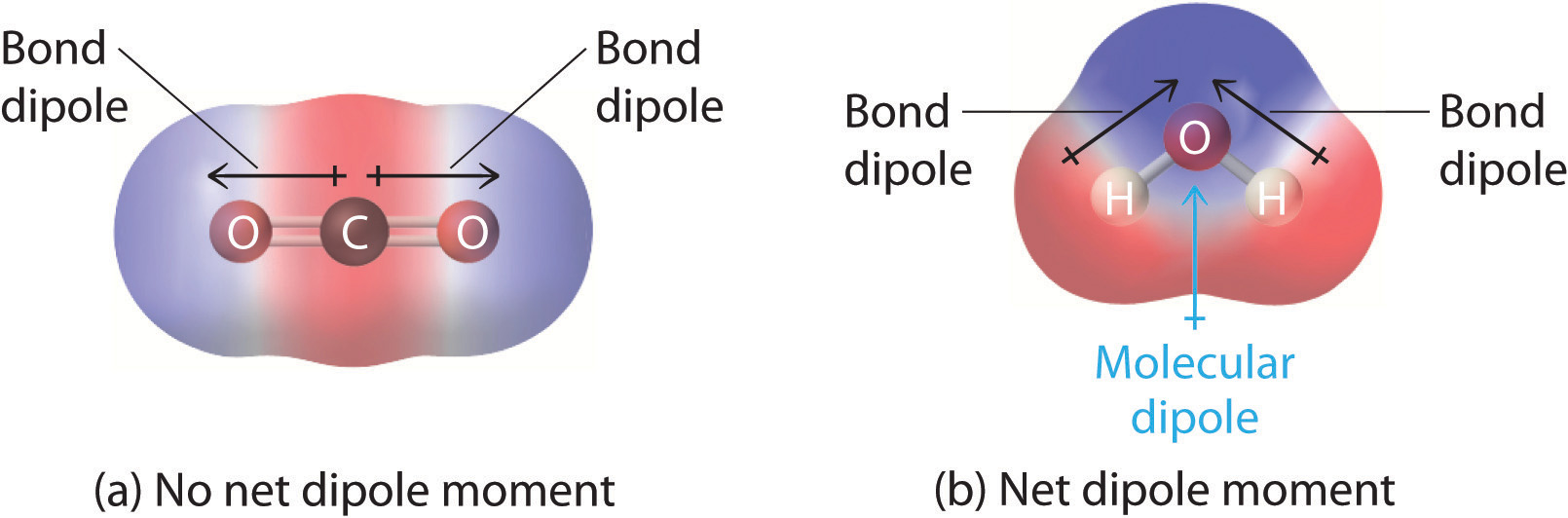
- Symmetrical shapes → dipoles cancel
- Asymmetrical shapes → dipoles do not cancel
Only the arrangement of atoms matters—not lone pairs—when naming polarity.
Key Rule for Molecular Polarity
A molecule is polar if:
- It contains at least one polar bond, and
- The molecular geometry is asymmetric
A molecule is nonpolar if bond dipoles cancel due to symmetry.
Polarity of Common Molecular Geometries
| Molecular Geometry | Typical Polarity | Reason |
|---|---|---|
| Linear (symmetrical) | Nonpolar | Bond dipoles cancel |
| Trigonal planar (symmetrical) | Nonpolar | Even electron distribution |
| Tetrahedral (symmetrical) | Nonpolar | Balanced dipole arrangement |
| Bent | Polar | Asymmetric geometry |
| Trigonal pyramidal | Polar | Lone pair creates asymmetry |
Evaluating Electron Distribution
When analyzing electron distribution:
- Identify the most electronegative atom(s)
- Determine bond dipole directions
- Assess whether dipoles cancel or reinforce
Net dipole moment ≠ 0 → molecule is polar.
Example
Carbon dioxide, CO₂, has polar C=O bonds. Determine whether the molecule is polar and justify your answer using molecular geometry.
▶️ Answer / Explanation
Carbon dioxide is nonpolar.
Although the C=O bonds are polar, the molecule has a linear geometry. The bond dipoles point in opposite directions and cancel, resulting in a symmetric electron distribution.
Example
A molecule has the formula NH₃. Use its Lewis diagram and molecular geometry to determine whether it is polar.
▶️ Answer / Explanation
Ammonia (NH₃) is polar.
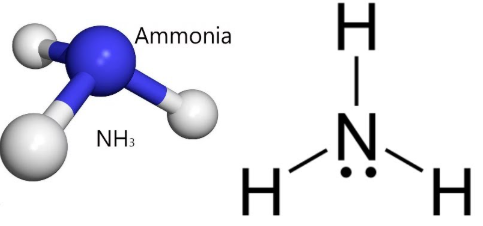
Nitrogen has a lone pair, giving the molecule a trigonal pyramidal geometry. The N–H bonds are polar, and their dipoles do not cancel due to asymmetry, resulting in a net dipole and a polar molecule.
