2.3A Types of Chemical Bonding- Pre AP Chemistry Study Notes - New Syllabus.
2.3A Types of Chemical Bonding- Pre AP Chemistry Study Notes
2.3A Types of Chemical Bonding- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
2.3.A.1 Create and/or evaluate a claim about the type of bonding in a compound based on its component elements and its macroscopic properties.
Key Concepts:
- 2.3.A Bonding between elements can be nonpolar covalent, polar covalent, or ionic.
2.3.A.1 — Bond Type Based on Elements and Macroscopic Properties
The type of bonding in a compound can be determined by examining the types of elements involved and the compound’s macroscopic (observable) properties. Bonding between elements may be nonpolar covalent, polar covalent, or ionic.
Bond type is explained by how electrons are shared or transferred due to electronegativity differences between atoms.
Electronegativity and Bond Type
Electronegativity is an atom’s ability to attract electrons. The difference in electronegativity between bonded atoms determines bond type.
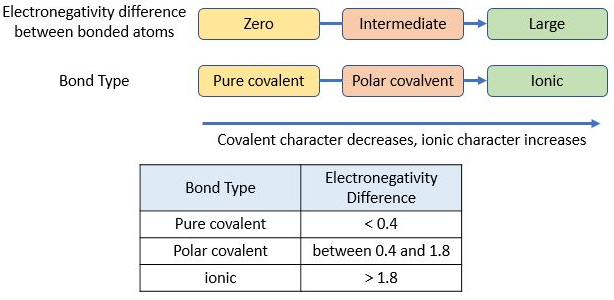
- Small difference → electrons shared nearly equally
- Moderate difference → electrons shared unequally
- Large difference → electrons transferred
Electron behavior at the particle level explains observable properties.
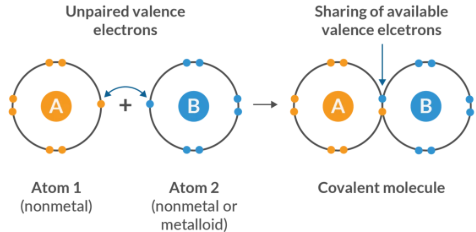
Nonpolar Covalent Bonding
Nonpolar covalent bonds occur when electrons are shared equally between atoms with similar electronegativities.
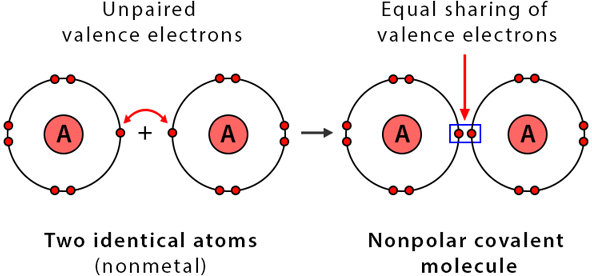
- Typically between identical or similar nonmetals
- No permanent partial charges
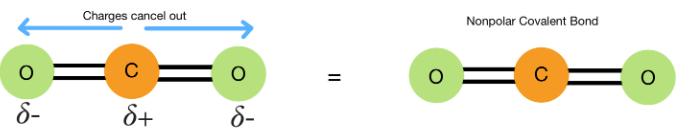
Macroscopic Properties
- Low melting and boiling points
- Often gases or liquids at room temperature
- Poor electrical conductivity
Polar Covalent Bonding
Polar covalent bonds occur when electrons are shared unequally due to a moderate electronegativity difference.
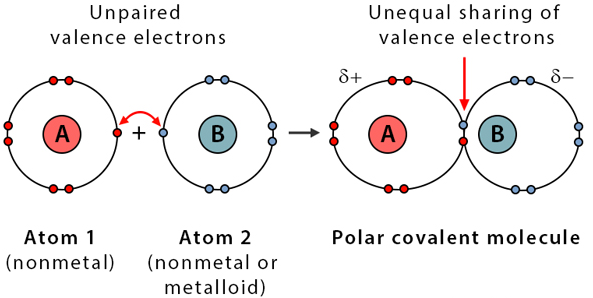
- Occurs between different nonmetals
- Partial positive and negative charges form
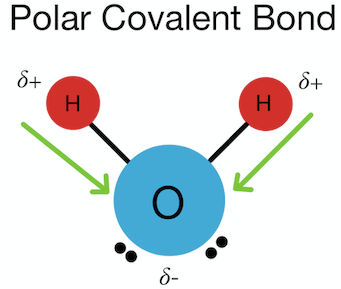
Macroscopic Properties
- Low to moderate melting points
- Often liquids or soft solids
- Poor conductivity as solids
- May dissolve in polar solvents
Ionic Bonding
Ionic bonds form when electrons are transferred from one atom to another, creating oppositely charged ions held together by electrostatic attraction.
- Usually between metals and nonmetals
- Large electronegativity difference
![]()
Macroscopic Properties
- High melting and boiling points
- Crystalline solids
- Conduct electricity when molten or dissolved in water
- Often soluble in water
Summary of Bond Types
| Bond Type | Electron Behavior | Typical Elements | Macroscopic Properties |
|---|---|---|---|
| Nonpolar covalent | Equal sharing | Nonmetal–nonmetal | Low mp/bp, poor conductivity |
| Polar covalent | Unequal sharing | Different nonmetals | Moderate mp/bp, polar behavior |
| Ionic | Electron transfer | Metal–nonmetal | High mp/bp, conductive when molten |
Creating and Evaluating Claims
A correct claim about bonding must:
- Identify the types of elements involved
- Relate electronegativity differences to electron behavior
- Use macroscopic properties as supporting evidence
Claims based on only one factor are incomplete.
Example
A compound is formed between two nonmetals and has a relatively low melting point. Create a claim identifying the bond type and justify your reasoning.
▶️ Answer / Explanation
The compound likely contains covalent bonds.
Bonding between nonmetals involves sharing electrons, and the low melting point supports covalent bonding rather than ionic bonding.
Example
A solid compound conducts electricity when dissolved in water and has a high melting point. One element is a metal and the other is a nonmetal. Create and evaluate a claim about the type of bonding present.
▶️ Answer / Explanation
The compound contains ionic bonding.
The metal–nonmetal combination indicates electron transfer. The high melting point and electrical conductivity in solution are characteristic macroscopic properties of ionic compounds.
