3.1A The Mole and Amount of Substance- Pre AP Chemistry Study Notes - New Syllabus.
3.1A The Mole and Amount of Substance- Pre AP Chemistry Study Notes
3.1A The Mole and Amount of Substance- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
3.1.A.1 Explain the relationship between the mass of a substance, the number of particles of that substance, and the number of moles of that substance.
3.1.A.2 Use the mole concept to calculate the mass, number of particles, or number of moles of a given substance.
Key Concepts:
- 3.1.A A large number of particles of a substance is needed to measure the physical properties of that substance.
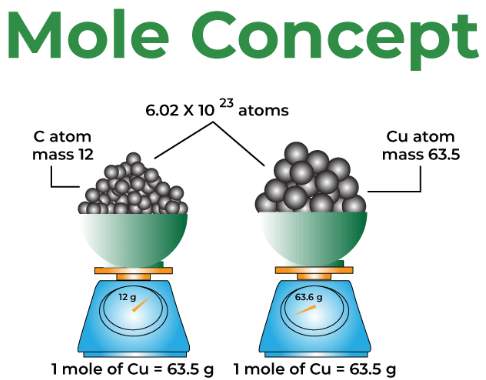
a. A mole of a substance contains Avogadro’s number (6.02 × 10²³) of particles.
b. The molar mass of an element listed on the periodic table is the mass, in grams, of a mole of atoms of that element.
3.1.A.1 — Mass, Moles, and Number of Particles
In chemistry, the mole is the bridge between the macroscopic world (measurable mass) and the microscopic world (atoms, molecules, or ions). The mass of a substance, the number of particles it contains, and the number of moles of that substance are all directly related.
Understanding this relationship allows chemists to count particles by measuring mass.
The Mole Concept
A mole is defined as a specific number of particles:![]()
\( \mathrm{1\ mol = 6.022 \times 10^{23}\ particles} \)
This number is called Avogadro’s number and applies to:
- Atoms
- Molecules
- Ions
One mole of any substance always contains the same number of particles, but the mass of one mole depends on the substance.
Molar Mass
Molar mass is the mass of one mole of a substance.
- Units: \( \mathrm{g\,mol^{-1}} \)
- Numerically equal to the atomic or molecular mass from the periodic table
For example, carbon has a molar mass of \( \mathrm{12.01\ g\,mol^{-1}} \), meaning one mole of carbon atoms has a mass of 12.01 g.
Relationship Between Mass and Moles
Mass and moles are related through molar mass:
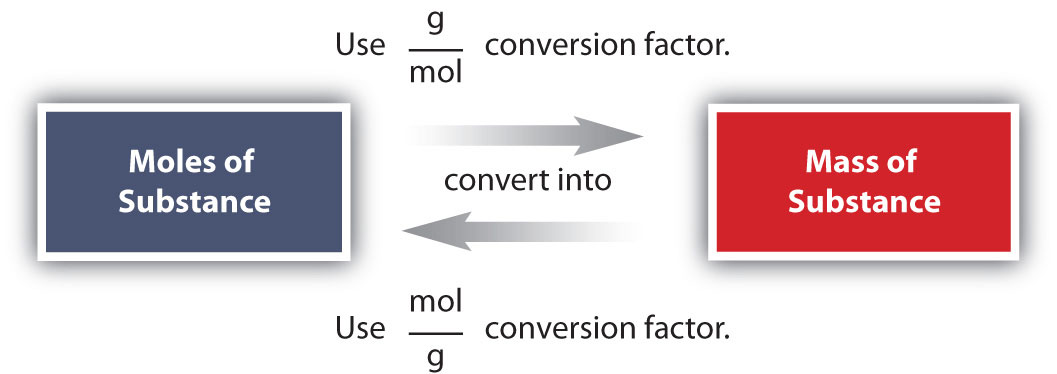
\( \mathrm{n = \dfrac{m}{M}} \)
- \( \mathrm{n} \) = number of moles
- \( \mathrm{m} \) = mass (g)
- \( \mathrm{M} \) = molar mass (\( \mathrm{g\,mol^{-1}} \))
Increasing the mass of a substance increases the number of moles proportionally.
Relationship Between Moles and Particles
Moles connect directly to the number of particles using Avogadro’s number:
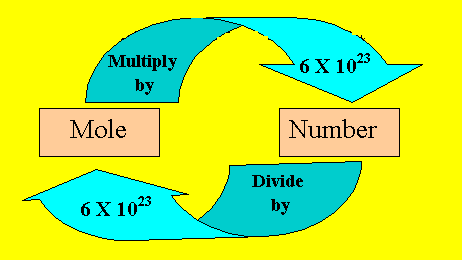
\( \mathrm{N = n \times 6.022 \times 10^{23}} \)
- \( \mathrm{N} \) = number of particles
- \( \mathrm{n} \) = number of moles
More moles always mean more particles.
Complete Mass–Mole–Particle Relationship
These three quantities are linked in a consistent chain:

- Mass ↔ moles (via molar mass)
- Moles ↔ particles (via Avogadro’s number)
Direct conversion between mass and particles always requires two steps: mass → moles → particles.
Summary of Key Relationships
| Quantity | Related Using |
|---|---|
| Mass ↔ Moles | Molar mass |
| Moles ↔ Particles | Avogadro’s number |
Creating and Evaluating Claims
A correct explanation must:
- Identify the mole as a counting unit
- Link mass to moles using molar mass
- Link moles to particles using Avogadro’s number
Statements that skip the mole step are incomplete.
Example
Calculate the number of moles in 18.0 g of water, H₂O.
▶️ Answer / Explanation
The molar mass of H₂O is \( \mathrm{18.0\ g\,mol^{-1}} \).
\( \mathrm{n = \dfrac{18.0}{18.0} = 1.00\ mol} \)
Example
How many molecules are present in 44.0 g of carbon dioxide, CO₂?
▶️ Answer / Explanation
Step 1: Calculate moles of CO₂.
Molar mass of CO₂ = \( \mathrm{44.0\ g\,mol^{-1}} \)
\( \mathrm{n = \dfrac{44.0}{44.0} = 1.00\ mol} \)
Step 2: Convert moles to particles.
\( \mathrm{N = 1.00 \times 6.022 \times 10^{23}} \)
\( \mathrm{N = 6.022 \times 10^{23}\ molecules} \)
3.1.A.2 — Calculations Using the Mole Concept
The mole concept allows chemists to calculate the mass, number of particles, or number of moles of a substance by using molar mass and Avogadro’s number.
All mole calculations rely on understanding that the mole is a counting unit that links the particle level to measurable mass.
Key Quantities in Mole Calculations
- Mass — measured in grams (g)
- Moles — amount of substance (mol)
- Particles — atoms, molecules, or ions
Conversions between these quantities always move through moles.
Step 1: Calculating Moles from Mass
To convert mass to moles, divide the mass by the molar mass.
\( \mathrm{n = \dfrac{m}{M}} \)
- \( \mathrm{n} \) = moles
- \( \mathrm{m} \) = mass in g
- \( \mathrm{M} \) = molar mass in \( \mathrm{g\,mol^{-1}} \)
This step is required whenever mass is given.
Step 2: Calculating Mass from Moles
To convert moles to mass, multiply by the molar mass.
\( \mathrm{m = nM} \)
This calculation determines how much a given amount of substance weighs.
Step 3: Calculating Particles from Moles
To calculate the number of particles, multiply moles by Avogadro’s number.
\( \mathrm{N = n \times 6.022 \times 10^{23}} \)
- \( \mathrm{N} \) = number of particles
- Particles may be atoms, molecules, or ions
Step 4: Calculating Moles from Particles
To convert particles to moles, divide by Avogadro’s number.
\( \mathrm{n = \dfrac{N}{6.022 \times 10^{23}}} \)
This conversion is required before finding mass from particles.
Two-Step Conversions
Direct conversions between mass and particles are not possible. They must always pass through moles:
- Mass → moles → particles
- Particles → moles → mass
Mole Conversion Summary
![]()
Creating and Evaluating Calculations
Accurate mole calculations must:
- Use the correct molar mass
- Include appropriate units
- Follow the correct conversion pathway
Skipping steps or incorrect units leads to incorrect results.
Example
Calculate the mass of 0.250 mol of sodium chloride, NaCl.
▶️ Answer / Explanation
Step 1: Determine molar mass of NaCl.
\( \mathrm{M = 22.99 + 35.45 = 58.44\ g\,mol^{-1}} \)
Step 2: Multiply by moles.
\( \mathrm{m = 0.250 \times 58.44} \)
\( \mathrm{m = 14.6\ g} \)
Example
How many oxygen atoms are present in 36.0 g of water, H₂O?
▶️ Answer / Explanation
Step 1: Calculate moles of H₂O.
\( \mathrm{M = 18.0\ g\,mol^{-1}} \)
\( \mathrm{n = \dfrac{36.0}{18.0} = 2.00\ mol} \)
Step 2: Convert moles to molecules.
\( \mathrm{2.00 \times 6.022 \times 10^{23} = 1.204 \times 10^{24}} \)
Step 3: Account for oxygen atoms (1 O per molecule).
\( \mathrm{1.204 \times 10^{24}\ oxygen\ atoms} \)
