3.2C Limiting Reactants- Pre AP Chemistry Study Notes - New Syllabus.
3.2C Limiting Reactants- Pre AP Chemistry Study Notes
3.2C Limiting Reactants- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
3.2.C.1 Create and/or evaluate models of a reaction mixture before and/or after a reaction has occurred, including situations with a limiting reactant.
Key Concepts:
- 3.2.C The limiting reactant is the reactant that is completely consumed during a chemical reaction. The limiting reactant determines the amount of product formed.
3.2.C.1 — Models of Reaction Mixtures and Limiting Reactants
A reaction mixture contains all reactant particles present before a chemical transformation and the products formed after the reaction occurs. Models of reaction mixtures help visualize how particles interact, how reactants are consumed, and how a limiting reactant controls the amount of product formed.
These models are especially useful for explaining situations in which one reactant is completely used up while another remains in excess.
Before a Chemical Reaction
In a particulate model before the reaction:
- Reactant particles are shown separately
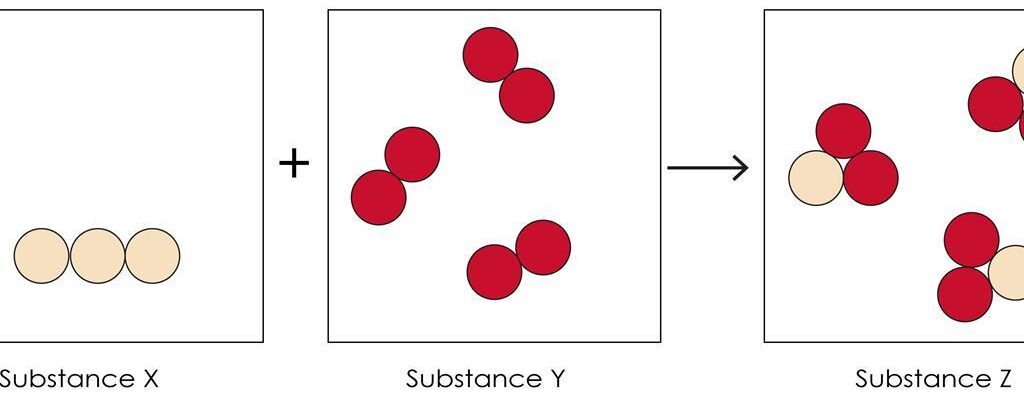
- Particles are present in specific ratios
- No products have formed yet
The relative numbers of reactant particles determine how much reaction can occur.
After a Chemical Reaction
In a particulate model after the reaction:
- Some reactant particles have rearranged into products
- One reactant may be completely consumed
- Excess reactant particles may remain unreacted
The number of product particles formed is fixed by the balanced equation.
The Limiting Reactant
The limiting reactant is the reactant that is: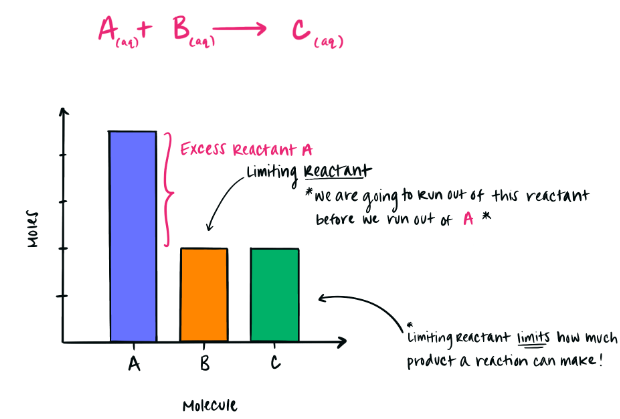
- Completely consumed during the reaction
- Present in insufficient quantity to react fully with other reactants
Once the limiting reactant is used up, the reaction stops.
Excess Reactant
Any reactant that remains after the limiting reactant is consumed is called the excess reactant.
- Excess reactant particles appear unchanged after the reaction
- They do not affect the maximum amount of product formed
Particle-Level Interpretation of Limiting Reactants
At the particle level:
- Only particles present in correct stoichiometric ratios can react
- Extra particles remain unused
- The limiting reactant determines how many product particles form
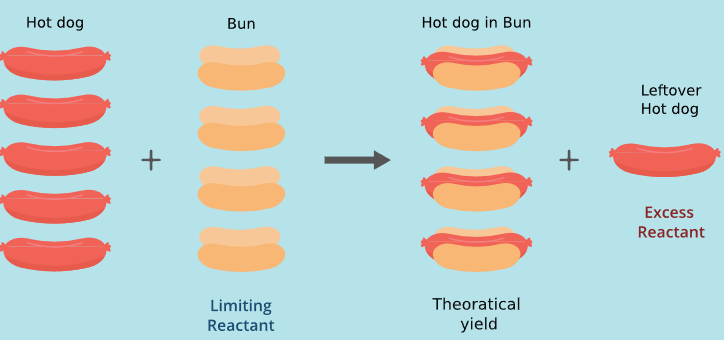
This explains why adding more excess reactant does not increase product yield.
Using Models to Identify the Limiting Reactant
To evaluate a reaction model:
- Count reactant particles before the reaction
- Compare their ratio to the balanced equation
- Identify which reactant runs out first
The reactant that disappears entirely is the limiting reactant.
Connecting Particulate Models and Equations
A correct reaction model must:
- Match the mole ratios in the balanced equation
- Show conservation of atoms
- Correctly identify limiting and excess reactants
Models that change atom counts or product ratios are incorrect.
Summary of Reaction Mixture Models
| Stage | What the Model Shows |
|---|---|
| Before reaction | Separate reactant particles |
| After reaction | Products + excess reactant |
| Limiting reactant | Completely consumed |
| Excess reactant | Remains unreacted |
Example
The reaction below occurs:
\( \mathrm{2H_2 + O_2 \rightarrow 2H_2O} \)
A particle model shows 4 hydrogen molecules and 1 oxygen molecule before the reaction. Identify the limiting reactant and describe the reaction mixture after the reaction.
▶️ Answer / Explanation
The balanced equation requires 2 hydrogen molecules for every 1 oxygen molecule.
With 1 oxygen molecule present, only 2 hydrogen molecules can react. Oxygen is the limiting reactant and will be completely consumed.
After the reaction, 2 water molecules form and 2 hydrogen molecules remain unreacted.
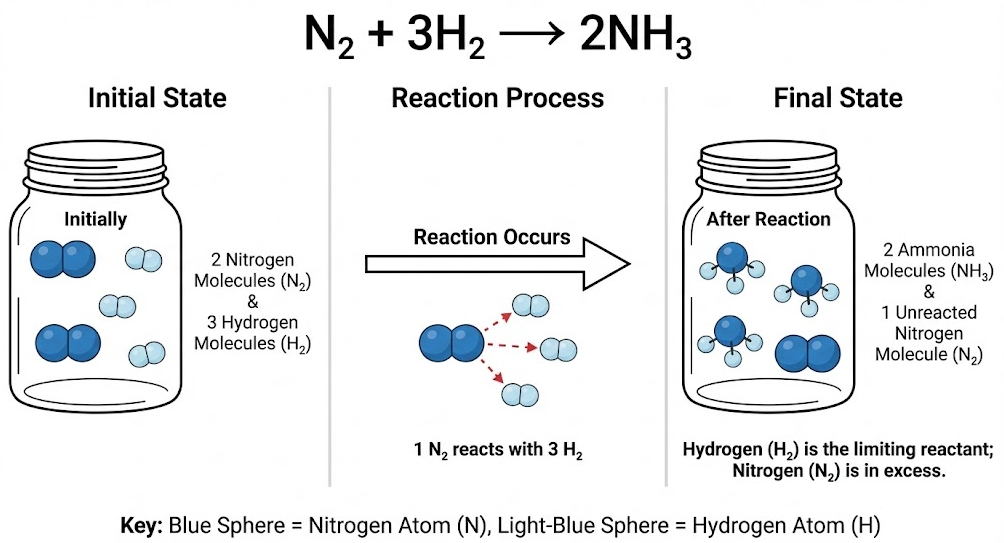
Example
Nitrogen reacts with hydrogen according to:
\( \mathrm{N_2 + 3H_2 \rightarrow 2NH_3} \)
A reaction mixture initially contains 2 nitrogen molecules and 3 hydrogen molecules. Create a particle-level description of the mixture after the reaction occurs.
▶️ Answer / Explanation
The equation requires 3 hydrogen molecules per 1 nitrogen molecule.
With only 3 hydrogen molecules available, hydrogen is the limiting reactant.
One nitrogen molecule reacts with all hydrogen molecules to form 2 ammonia molecules.
After the reaction, 2 ammonia molecules and 1 unreacted nitrogen molecule remain.