3.2D Theoretical and Percent Yield- Pre AP Chemistry Study Notes - New Syllabus.
3.2D Theoretical and Percent Yield- Pre AP Chemistry Study Notes
3.2D Theoretical and Percent Yield- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
3.2.D.1 Calculate the theoretical yield and/or percent yield of a chemical reaction.
Key Concepts:
- 3.2.D A balanced chemical reaction equation, combined with the mole concept, can be used to calculate the theoretical and percent yield of a reaction.
3.2.D.1 — Theoretical Yield and Percent Yield
In a chemical reaction, the balanced chemical equation and the mole concept are used to predict how much product should form. This predicted amount is called the theoretical yield.
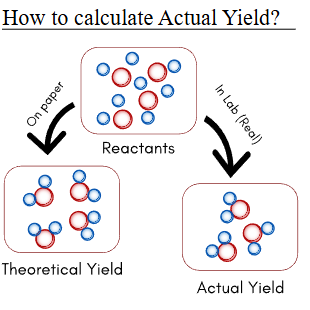
In real reactions, the amount of product actually collected may be less. Comparing the predicted amount to the measured amount allows chemists to calculate the percent yield of a reaction.
Theoretical Yield
The theoretical yield is the maximum amount of product that can be formed from given reactants assuming:
- The reaction goes to completion
- The limiting reactant is completely consumed
- No side reactions occur
- No product is lost during collection
Theoretical yield is calculated using stoichiometry and depends only on the limiting reactant.
Actual Yield
The actual yield is the amount of product actually obtained from an experiment.
- Measured experimentally
- Usually less than the theoretical yield
Differences arise due to incomplete reactions, product loss, or competing reactions.
Calculating Theoretical Yield
Steps to calculate theoretical yield:
- Write and balance the chemical equation
- Identify the limiting reactant
- Convert the given amount of limiting reactant to moles
- Use mole ratios to find moles of product
- Convert moles of product to mass (if needed)
The result represents the maximum possible product.
Percent Yield
Percent yield compares the efficiency of a reaction by measuring how much product was actually formed relative to the theoretical maximum.

Percent yield reflects how effective a reaction is under real conditions.
Interpreting Percent Yield Values
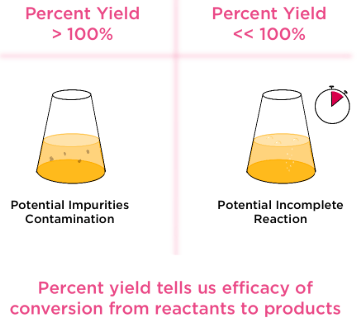
- 100% → ideal reaction (theoretical case)
- Less than 100% → expected for real reactions
- Greater than 100% → indicates experimental error
Yields above 100% often result from impurities or measurement mistakes.
Particle-Level Explanation
At the particle level:
- The limiting reactant controls the number of product particles formed
- Not all collisions produce product
- Some product particles may be lost during handling
This explains why actual yield is often lower than theoretical yield.
Summary of Yield Concepts
| Term | Meaning |
|---|---|
| Theoretical yield | Maximum possible product |
| Actual yield | Product obtained experimentally |
| Percent yield | Reaction efficiency |
Evaluating Yield Calculations
Correct yield calculations must:
- Use a balanced chemical equation
- Base calculations on the limiting reactant
- Use correct mole ratios and molar masses
- Clearly distinguish actual vs theoretical yield
Example
Calcium oxide forms according to the reaction:
\( \mathrm{CaCO_3 \rightarrow CaO + CO_2} \)
Calculate the theoretical yield of CaO produced from 50.0 g of CaCO₃.
▶️ Answer / Explanation
Step 1: Convert mass of CaCO₃ to moles.
\( \mathrm{M = 100.1\ g\,mol^{-1}} \)
\( \mathrm{n = \dfrac{50.0}{100.1} = 0.500\ mol} \)
Step 2: Use mole ratio (1:1).
\( \mathrm{n_{CaO} = 0.500\ mol} \)
Step 3: Convert moles of CaO to mass.
\( \mathrm{M_{CaO} = 56.1\ g\,mol^{-1}} \)
\( \mathrm{m = 0.500 \times 56.1 = 28.1\ g} \)
Example
Zinc reacts with hydrochloric acid according to:
\( \mathrm{Zn + 2HCl \rightarrow ZnCl_2 + H_2} \)
If the theoretical yield of ZnCl₂ is 68.0 g but only 54.4 g is obtained, calculate the percent yield.
▶️ Answer / Explanation
Use the percent yield formula.
\( \mathrm{\%\ yield = \dfrac{54.4}{68.0} \times 100} \)
\( \mathrm{\%\ yield = 80.0\%} \)
