4.1A Precipitation Reactions- Pre AP Chemistry Study Notes - New Syllabus.
4.1A Precipitation Reactions- Pre AP Chemistry Study Notes
4.1A Precipitation Reactions- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
4.1.A.1 Predict the products of a precipitation reaction.
Key Concepts:
- 4.1.A Precipitation reactions may occur when two aqueous solutions are mixed, because some ionic compounds are insoluble in water and therefore precipitate out of solution.
4.1.A.1 — Predicting the Products of a Precipitation Reaction
A precipitation reaction occurs when two aqueous ionic solutions are mixed and an insoluble ionic compound forms. This insoluble solid is called a precipitate and separates from the solution.
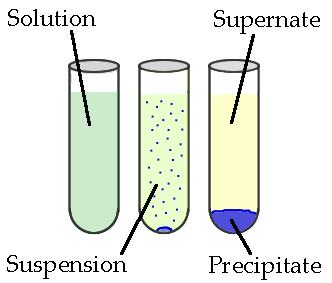
Precipitation reactions can be predicted by applying solubility rules and understanding how ions rearrange in solution.
Particle-Level Description
When an ionic compound dissolves in water, it dissociates into ions:
\( \mathrm{NaCl(s) \rightarrow Na^+(aq) + Cl^-(aq)} \)
When two aqueous solutions are mixed:
- All ions are free to move in solution
- Ions may recombine in new ways
- If a combination of ions forms an insoluble compound, a precipitate forms
Key Idea Behind Precipitation Reactions
Not all ionic compounds are soluble in water.
If ion exchange produces an insoluble salt, that compound will precipitate out of solution.
Steps to Predict Products of a Precipitation Reaction
- Write the formulas of the two aqueous ionic compounds
- Separate each compound into its ions
- Swap the ions to form two new ionic compounds (double replacement)
- Use solubility rules to determine if either product is insoluble
If one product is insoluble, it is written as a solid (s) and is the precipitate.
Solubility Rules (Pre-AP Focus)
- All nitrates (\( \mathrm{NO_3^-} \)) are soluble
- All Group 1 metal salts are soluble
- Most chlorides are soluble (except with Ag⁺, Pb²⁺)
- Most sulfates are soluble (except with Ba²⁺, Pb²⁺)
- Most carbonates and hydroxides are insoluble
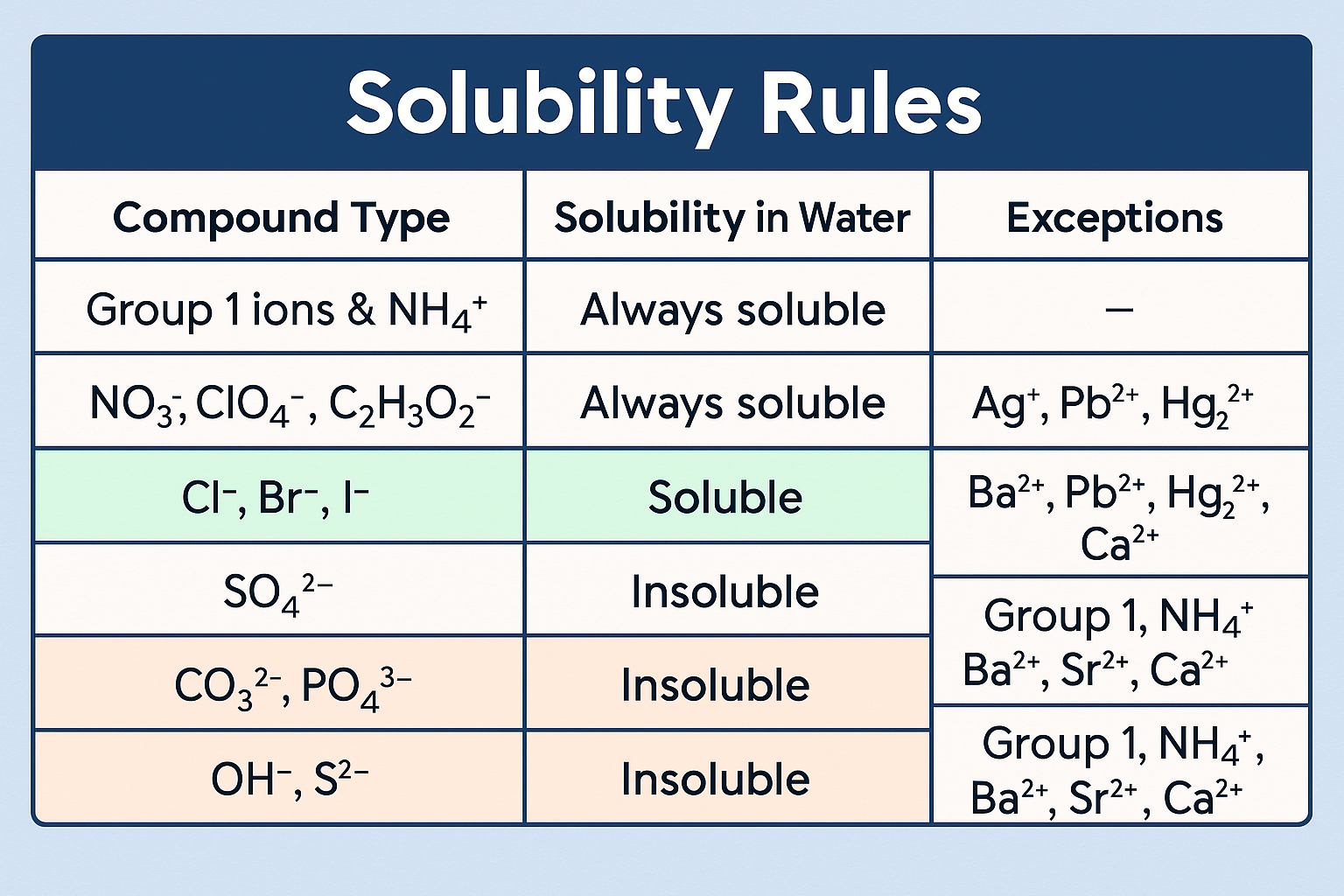
Only one insoluble product is needed for a precipitation reaction to occur.
Molecular Equation
The molecular equation shows compounds as whole units:
\( \mathrm{AgNO_3(aq) + NaCl(aq) \rightarrow AgCl(s) + NaNO_3(aq)} \)
The solid \( \mathrm{AgCl(s)} \) is the precipitate.
Total Ionic and Net Ionic Equations
Total ionic equation (shows all ions in solution):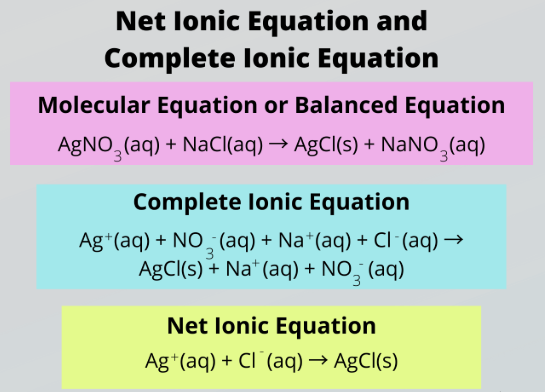
\( \mathrm{Ag^+(aq) + NO_3^-(aq) + Na^+(aq) + Cl^-(aq) \rightarrow AgCl(s) + Na^+(aq) + NO_3^-(aq)} \)
Net ionic equation (shows only reacting ions):
\( \mathrm{Ag^+(aq) + Cl^-(aq) \rightarrow AgCl(s)} \)
Spectator ions are omitted because they do not participate in the reaction.
When No Precipitation Occurs
If both possible products are soluble according to solubility rules, no reaction occurs.

All ions remain in solution, and no precipitate forms.
Evaluating Claims About Precipitation
A valid claim must:
- Identify the ions present in solution
- Apply solubility rules correctly
- Explain why a solid does or does not form
Claims that name a precipitate without justification are incomplete.
Example
Predict the products when aqueous solutions of barium chloride and sodium sulfate are mixed.
▶️ Answer / Explanation
The ions present are \( \mathrm{Ba^{2+}, Cl^-, Na^+,} \) and \( \mathrm{SO_4^{2-}} \).
Swapping ions gives barium sulfate and sodium chloride.
Barium sulfate is insoluble, so a precipitate forms:
\( \mathrm{BaCl_2(aq) + Na_2SO_4(aq) \rightarrow BaSO_4(s) + 2NaCl(aq)} \)
Example
Two clear aqueous solutions are mixed and a white solid forms. The reactants are potassium carbonate and calcium nitrate. Predict the products and write the net ionic equation.
▶️ Answer / Explanation
Carbonates are generally insoluble except with Group 1 metals.
The precipitate is calcium carbonate:
\( \mathrm{K_2CO_3(aq) + Ca(NO_3)_2(aq) \rightarrow CaCO_3(s) + 2KNO_3(aq)} \)
Net ionic equation:
\( \mathrm{Ca^{2+}(aq) + CO_3^{2-}(aq) \rightarrow CaCO_3(s)} \)
