4.1B Representations of Precipitation Reactions- Pre AP Chemistry Study Notes - New Syllabus.
4.1B Representations of Precipitation Reactions- Pre AP Chemistry Study Notes
4.1B Representations of Precipitation Reactions- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
4.1.B.1 Create and/or evaluate models of precipitation reactions.
Key Concepts:
- 4.1.B Precipitation reactions can be modeled by molecular equations, net ionic equations, and particulate representations.
4.1.B.1 — Modeling Precipitation Reactions
Precipitation reactions can be represented using multiple complementary models. Each model highlights a different level of chemical understanding and helps explain how and why a precipitate forms when two aqueous ionic solutions are mixed.

According to this standard, precipitation reactions are modeled using:
- Molecular equations
- Total ionic equations
- Net ionic equations
- Particulate (particle-level) representations
Why Multiple Models Are Used
Each model answers a different question:
- Molecular equation → What substances are mixed and formed?
- Total ionic equation → Which ions are present in solution?
- Net ionic equation → Which ions actually react?
- Particulate model → What is happening at the particle level?
All models must be consistent with one another.
Molecular Equation Model
The molecular equation shows reactants and products as intact ionic compounds, including physical states.
Example:
\( \mathrm{AgNO_3(aq) + NaCl(aq) \rightarrow AgCl(s) + NaNO_3(aq)} \)
This model shows:
- Two aqueous solutions are mixed
- A solid precipitate forms
- One product remains dissolved
Total Ionic Equation Model
The total ionic equation represents all soluble ionic compounds as dissociated ions in solution.
Example:
\( \mathrm{Ag^+(aq) + NO_3^-(aq) + Na^+(aq) + Cl^-(aq) \rightarrow AgCl(s) + Na^+(aq) + NO_3^-(aq)} \)
This model makes it clear which ions are freely moving in solution.
Spectator Ions
Spectator ions are ions that:
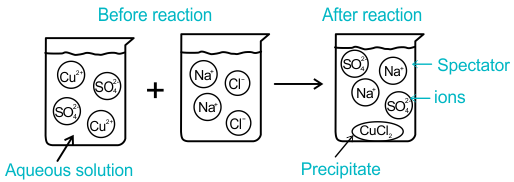
- Appear unchanged on both sides of the equation
- Do not participate in precipitate formation
In the example above, \( \mathrm{Na^+} \) and \( \mathrm{NO_3^-} \) are spectator ions.
Net Ionic Equation Model
The net ionic equation shows only the ions directly involved in forming the precipitate.
\( \mathrm{Ag^+(aq) + Cl^-(aq) \rightarrow AgCl(s)} \)
This model emphasizes the essential chemical change and removes unnecessary information.
Particulate (Particle-Level) Models
Particulate models show individual ions and particles before and after mixing. They help visualize:
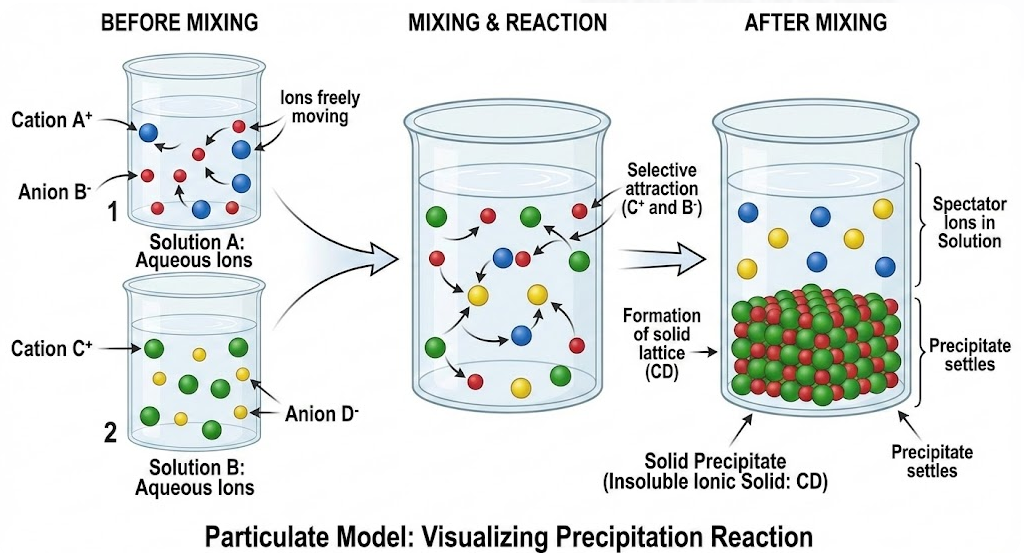
- Ions freely moving in solution before reaction
- Selective attraction between specific ions
- Formation of a solid lattice as ions join together
A correct particulate model must show that:
- Spectator ions remain dispersed in solution
- Only insoluble ion pairs form a solid
- The precipitate settles out of solution
Evaluating Precipitation Reaction Models
To evaluate a model of a precipitation reaction, check that:
- Solubility rules are applied correctly
- Physical states match the reaction
- Spectator ions are not included in the net ionic equation
- Particle diagrams match the symbolic equations
Any inconsistency indicates an incorrect model.
Example
Write the molecular, total ionic, and net ionic equations for the reaction between aqueous lead(II) nitrate and potassium iodide.
▶️ Answer / Explanation
Molecular equation:
\( \mathrm{Pb(NO_3)_2(aq) + 2KI(aq) \rightarrow PbI_2(s) + 2KNO_3(aq)} \)
Total ionic equation:
\( \mathrm{Pb^{2+}(aq) + 2NO_3^-(aq) + 2K^+(aq) + 2I^-(aq) \rightarrow PbI_2(s) + 2K^+(aq) + 2NO_3^-(aq)} \)
Net ionic equation:
\( \mathrm{Pb^{2+}(aq) + 2I^-(aq) \rightarrow PbI_2(s)} \)
Example
A student draws a particulate model showing all ions clumping together after two aqueous solutions are mixed. Evaluate this model and explain whether it correctly represents a precipitation reaction.
▶️ Answer / Explanation
The model is incorrect.
In a precipitation reaction, only the ions that form an insoluble compound should cluster together as a solid. Spectator ions must remain separated and dispersed in solution.
