4.3D Representations of Acid–Base Reactions- Pre AP Chemistry Study Notes - New Syllabus.
4.3D Representations of Acid–Base Reactions- Pre AP Chemistry Study Notes
4.3D Representations of Acid–Base Reactions- Pre AP Chemistry Study Notes – New Syllabus.
LEARNING OBJECTIVE
4.3.D.1 Create and/or evaluate models of a reaction between a strong acid and a strong base.
Key Concepts:
- 4.3.D Acid–base reactions can be modeled by molecular equations, net ionic equations, and particulate representations.
4.3.D.1 — Modeling Strong Acid–Strong Base Reactions
Reactions between a strong acid and a strong base are best understood by using multiple complementary models. Each model highlights a different level of explanation while describing the same chemical process—the transfer of a hydrogen ion that produces water and an aqueous ionic compound (salt).
Why Multiple Models Are Used
Strong acid–strong base reactions involve processes that are not fully visible from a single representation. Therefore, scientists use:
- Molecular equations to show substances reacting
- Net ionic equations to focus on reacting species
- Particulate models to show particle behavior in solution
All valid models must be consistent with one another.
Molecular Equation Model
The molecular equation shows all reactants and products as complete chemical formulas.
Example:
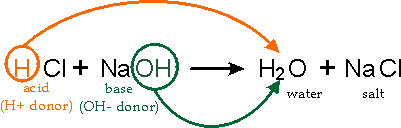
This model shows:
- Reacting acid and base
- Formation of water
- Formation of a dissolved ionic compound
However, it does not show which particles actually react.
Complete Ionic Equation Model
The complete ionic equation breaks all strong electrolytes into their ions.
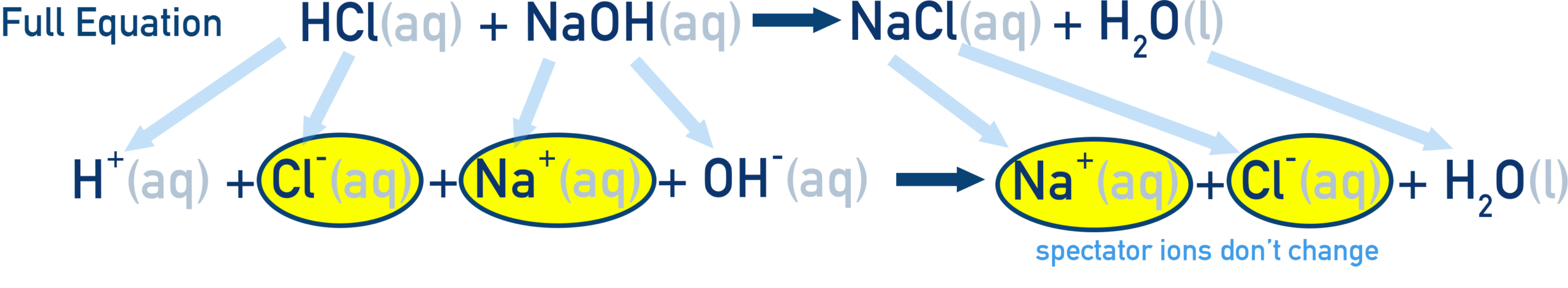
This model reveals:
- Which ions participate directly
- Which ions are spectators
Net Ionic Equation Model
The net ionic equation removes spectator ions and shows only the particles involved in the reaction.
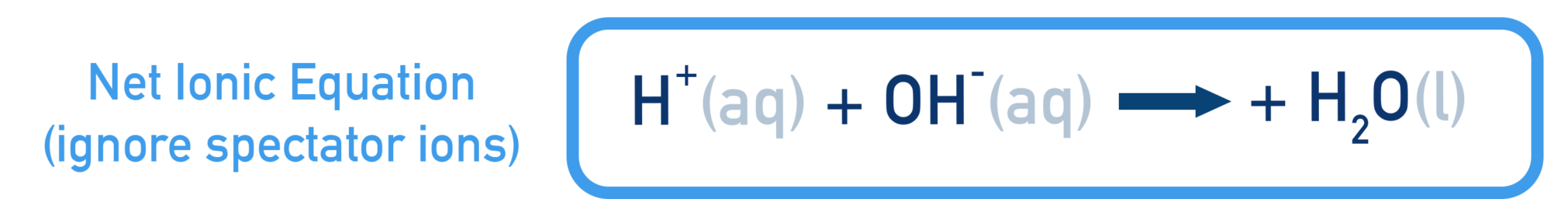
This equation:
- Represents all strong acid–strong base reactions
- Shows direct hydrogen-ion transfer
- Highlights water formation as the key process
Particulate (Particle-Level) Model
Particulate models show what happens at the level of individual particles.
A correct particulate model must show:
![]()
- \( \mathrm{H^+} \) ions and \( \mathrm{OH^-} \) ions colliding
- Formation of \( \mathrm{H_2O} \) molecules
- Spectator ions remaining unchanged and dispersed
No undissociated acid or base particles should appear, because both are strong and fully dissociated.
Consistency Across All Models
All three models must agree on:
- Which species react
- The formation of water
- The presence of spectator ions
If any model contradicts the others, it is incorrect.
Evaluating Acid–Base Reaction Models
When evaluating a model, check whether it:
- Shows complete dissociation of strong acids and bases
- Uses the correct net ionic equation
- Correctly identifies spectator ions
- Accurately represents water formation
Models showing molecules instead of ions for strong substances are scientifically inaccurate.
Example
Write the molecular, complete ionic, and net ionic equations for the reaction between hydrochloric acid and potassium hydroxide.
▶️ Answer / Explanation
Molecular:
\( \mathrm{HCl(aq) + KOH(aq) \rightarrow KCl(aq) + H_2O(l)} \)
Complete ionic:
\( \mathrm{H^+ + Cl^- + K^+ + OH^- \rightarrow K^+ + Cl^- + H_2O} \)
Net ionic:
\( \mathrm{H^+ + OH^- \rightarrow H_2O} \)
Example
A particulate model of a strong acid–strong base reaction shows several intact acid molecules remaining after the reaction. Evaluate this model.
▶️ Answer / Explanation
The model is incorrect.
Strong acids and strong bases dissociate completely in water. A correct particulate model should show only ions before the reaction and water molecules forming during the reaction.
Intact acid molecules should not appear.
