Pre AP Chemistry -1.2B Endothermic and Exothermic Processes- MCQ Exam Style Questions -New Syllabus 2025-2026
Pre AP Chemistry -1.2B Endothermic and Exothermic Processes- MCQ Exam Style Questions – New Syllabus 2025-2026
Pre AP Chemistry -1.2B Endothermic and Exothermic Processes- MCQ Exam Style Questions – Pre AP Chemistry – per latest Pre AP Chemistry Syllabus.
The table shows the initial and final temperatures for four different reactions.
| reaction | initial temperature/°C | final temperature/°C |
|---|---|---|
| 1 | 19 | 28 |
| 2 | 18 | 16 |
| 3 | 20 | 20 |
| 4 | 18 | 19 |
Which reactions are endothermic?
A) 1 and 4
B) 2 and 3
C) 2 only
D) 4 only
▶️ Answer/Explanation
Ans: C
An endothermic reaction absorbs heat from the surroundings, causing the temperature to decrease. Only reaction 2 shows a temperature decrease (from 18°C to 16°C). Reaction 1 shows a temperature increase (exothermic), reaction 3 shows no change (possibly no reaction or perfect insulation), and reaction 4 shows a slight increase (exothermic). Therefore, only reaction 2 is endothermic.
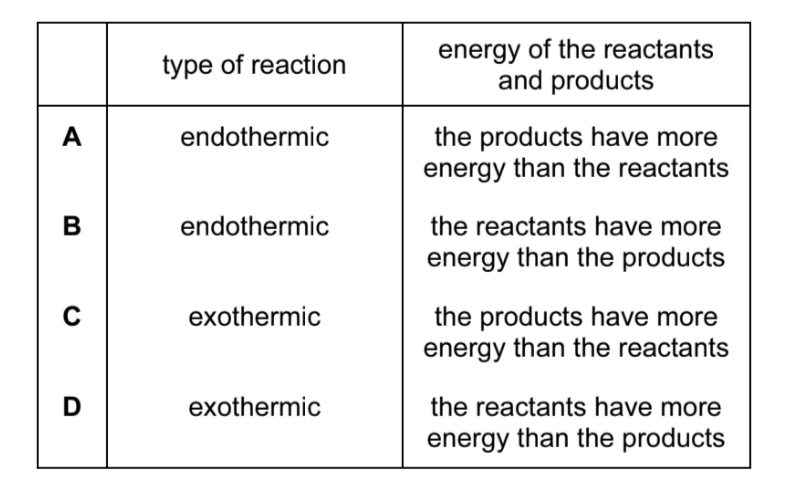
Which row describes the reaction pathway diagram and energy change in an exothermic reaction?
| reaction pathway diagram | energy is | |
|---|---|---|
| A | reactants higher than products | absorbed |
| B | reactants higher than products | released |
| C | reactants lower than products | absorbed |
| D | reactants lower than products | released |
▶️ Answer/Explanation
Ans: B
In an exothermic reaction, the reactants have more energy than the products, so the reaction pathway diagram shows reactants at a higher energy level than products. Energy is released to the surroundings in an exothermic reaction (often as heat). Therefore, the correct combination is: reactants higher than products and energy is released. Option A describes an endothermic reaction where energy is absorbed, and options C and D have the energy levels reversed.
The temperature of the water in two beakers, X and Y, is measured as 21.5°C.
5 g of sodium chloride is dissolved in the water in beaker X. The temperature changes to 18.0°C.
5 g of calcium oxide is dissolved in the water in beaker Y. The temperature changes to 29.4°C.
Which types of process are occurring in beakers X and Y?
| X | Y | |
|---|---|---|
| A | endothermic | endothermic |
| B | endothermic | exothermic |
| C | exothermic | endothermic |
| D | exothermic | exothermic |
▶️ Answer/Explanation
Ans: B
To determine whether the processes are endothermic or exothermic:
Beaker X (sodium chloride):
The temperature decreases from 21.5°C to 18.0°C, indicating heat is absorbed from the surroundings. This is characteristic of an endothermic process.
Beaker Y (calcium oxide):
The temperature increases from 21.5°C to 29.4°C, indicating heat is released to the surroundings. This is characteristic of an exothermic process.
This matches option B: X is endothermic and Y is exothermic.
The diagram shows a match.
By striking the match, a chemical reaction takes place.
Which row describes the chemical reaction?
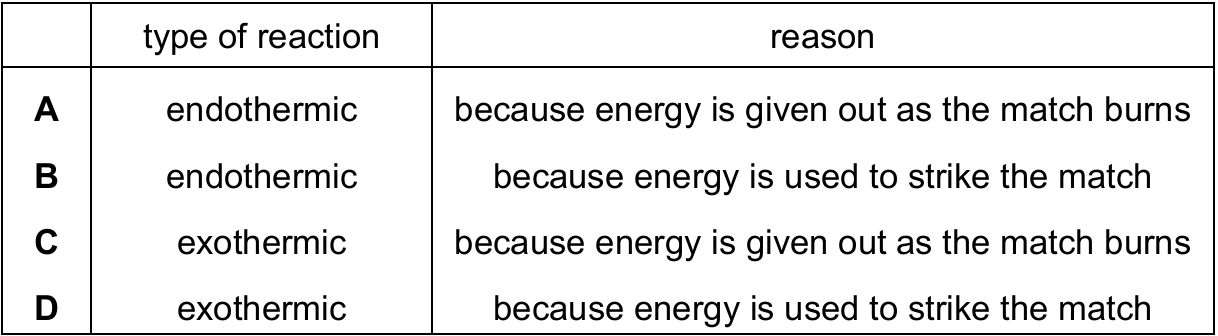
▶️ Answer/Explanation
Ans: C
The burning of a match is a classic example of an exothermic reaction because:
1. Heat and light energy are released during the combustion process
2. The energy required to initiate the reaction (striking the match) is different from the energy change of the reaction itself
3. The correct reason is that energy is given out as the match burns, not that energy is used to strike it
Therefore, option C correctly identifies the reaction as exothermic with the proper explanation.
Which reaction pathway diagram represents an endothermic reaction?
A) 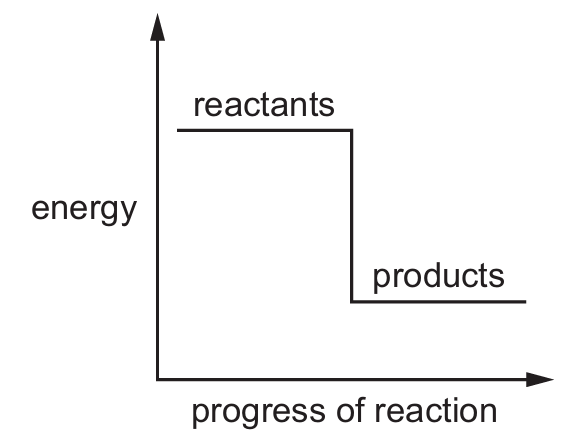
B) 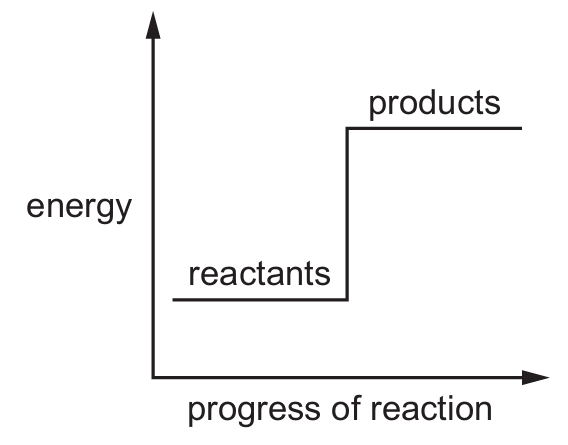
C) 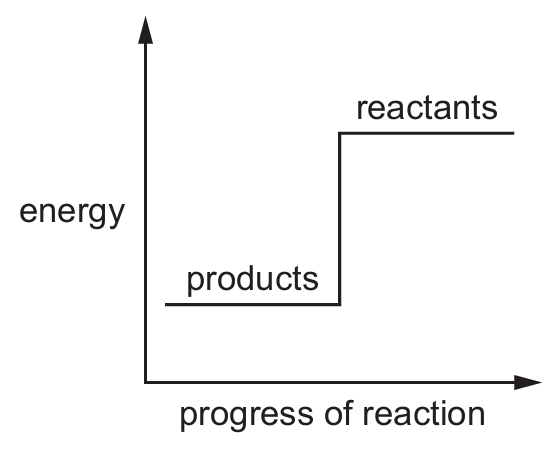
D) 
▶️ Answer/Explanation
Ans: B
An endothermic reaction is one that absorbs energy from its surroundings. In terms of reaction pathway diagrams:
– The products will be at a higher energy level than the reactants
– There will be an upward slope from reactants to products
– The difference in energy represents the energy absorbed
Option B shows this characteristic pattern where the products have higher energy than the reactants, indicating energy was absorbed during the reaction.
Which statements about endothermic reactions are correct?
- The energy of the products is greater than the energy of the reactants.
- The energy of the reactants is greater than the energy of the products.
- The temperature of the surroundings increases during the reaction.
- The temperature of the surroundings decreases during the reaction.
A) 1 and 3
B) 1 and 4
C) 2 and 3
D) 2 and 4
▶️ Answer/Explanation
Ans: B
In an endothermic reaction:
1. The products have higher energy than the reactants because energy is absorbed (statement 1 is correct).
2. The surroundings lose energy, causing a decrease in temperature (statement 4 is correct).
3. Statements 2 and 3 are incorrect because the reactants do not have higher energy, and the surroundings cool down, not heat up.
Therefore, the correct combination is 1 and 4 (Option B).
Three reactions are described.
- An acid is added to substance H. Rapid fizzing happens and the temperature decreases.
- When substance J is ignited, it produces large quantities of heat.
- Substance K reacts slowly with air and becomes warmer.
Which reactions are endothermic?
A) 1, 2 and 3
B) 1 and 2 only
C) 1 only
D) 2 and 3 only
▶️ Answer/Explanation
Ans: C
An endothermic reaction absorbs heat from the surroundings, causing a temperature decrease. Only reaction 1 shows this behavior. Reaction 2 is exothermic (heat produced), and reaction 3 is exothermic (temperature increases). Therefore, the correct answer is C (1 only).
Which row describes what happens during an endothermic reaction?
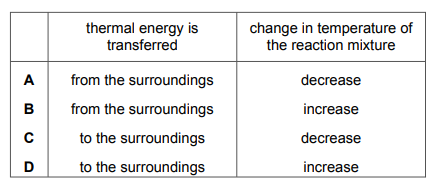
▶️ Answer/Explanation
Ans: A
An endothermic reaction absorbs heat from the surroundings, resulting in a decrease in temperature. The energy of the products is higher than the reactants because energy is taken in. Therefore, the correct row is A, where energy is absorbed (\(\Delta H > 0\)) and the temperature of the surroundings decreases.
When calcium carbonate is heated strongly, carbon dioxide gas is produced. Which words describe the type of change that occurs?
A) endothermic and chemical
B) endothermic and physical
C) exothermic and chemical
D) exothermic and physical
▶️ Answer/Explanation
Ans: A
When calcium carbonate (\( \text{CaCO}_3 \)) is heated, it decomposes into calcium oxide (\( \text{CaO} \)) and carbon dioxide (\( \text{CO}_2 \)). This is a chemical change because new substances are formed. The reaction requires heat energy to proceed, making it endothermic. Thus, the correct description is endothermic and chemical.
Which row describes the changes that occur in an endothermic reaction?

▶️ Answer/Explanation
Ans: C
An endothermic reaction absorbs heat (\(\Delta H > 0\)) from the surroundings, causing the temperature of the surroundings to decrease. The products have higher energy than the reactants, as energy is taken in. Therefore, the correct description is given in row C, where energy is absorbed and the surroundings cool down.
Which change of state is an exothermic process?
A. condensation
B. evaporation
C. melting
D. sublimation
▶️ Answer/Explanation
Ans: A
Condensation is an exothermic process because energy is released when gas molecules transition to the liquid phase. During condensation, intermolecular forces strengthen as molecules come closer together, releasing heat to the surroundings. In contrast, evaporation, melting, and sublimation are endothermic processes as they require energy input to overcome intermolecular forces.
The energy level diagram shows the energy of the reactants and products in a chemical reaction.

Which row correctly describes the energy change and the type of reaction shown?

▶️ Answer/Explanation
Ans: B
From the energy level diagram, the products have lower energy than the reactants, indicating energy is released. This defines an exothermic reaction. The energy change is negative (since energy is lost to the surroundings), and the reaction type is exothermic. Thus, the correct row is B.
Sodium nitrate is added to water in a beaker and stirred until it dissolves.
At the end of the experiment, the beaker feels cold.
Which row describes the reaction?

▶️ Answer/Explanation
Ans: A
When sodium nitrate dissolves in water, the beaker feels cold because the process is endothermic. This means energy is absorbed from the surroundings to break the ionic bonds in the solid sodium nitrate. As a result, the temperature of the solution decreases, confirming that the reaction is endothermic (energy is taken in). The correct row in the table is the one describing an endothermic process where energy is absorbed.
Which changes occur when hydrogen is burned in oxygen?
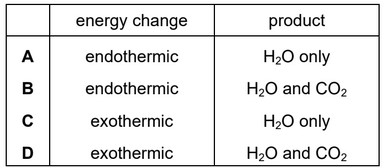
▶️ Answer/Explanation
Ans: C
The combustion of hydrogen in oxygen is an exothermic reaction, meaning it releases energy. The balanced chemical equation is:
\[ 2H_2(g) + O_2(g) \rightarrow 2H_2O(l) + \text{Energy} \]
Key observations:
- Energy is released as heat and light (exothermic).
- Water (\(H_2O\)) is formed as the product.
- The reaction involves breaking \(H-H\) and \(O=O\) bonds and forming new \(O-H\) bonds.
Thus, the correct answer is C, as the reaction is exothermic and produces water.
The complete combustion of propane is exothermic.
The equation for this reaction is shown.
\(C_3H_8 + 5O_2 \rightarrow 3CO_2 + 4H_2O\)
Which energy level diagram represents the complete combustion of propane?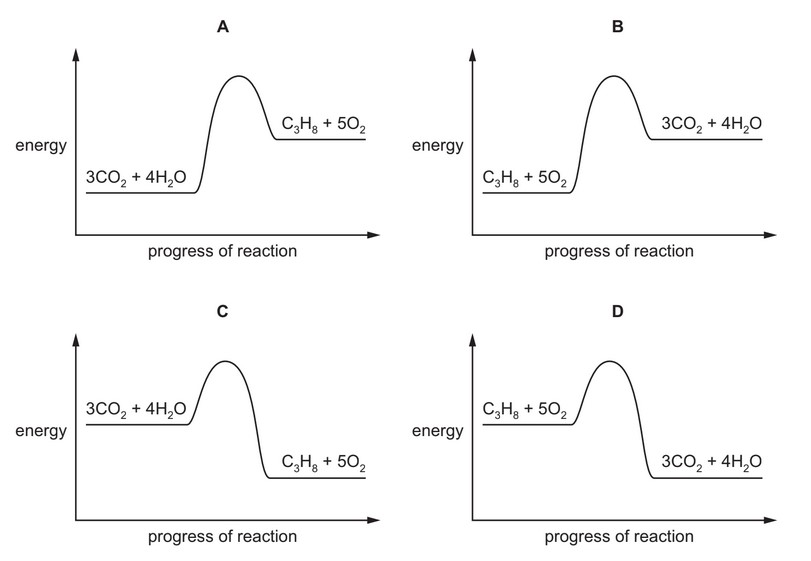
▶️ Answer/Explanation
Ans: D
The combustion of propane (\(C_3H_8\)) is exothermic, meaning energy is released. In an energy level diagram:
- The reactants (\(C_3H_8 + 5O_2\)) must be at a higher energy level than the products (\(3CO_2 + 4H_2O\)).
- The difference in energy is released as heat, represented by a downward slope in the diagram.
Only diagram D correctly shows the reactants at a higher energy level than the products, with energy released to the surroundings.
Which row describes an endothermic reaction?
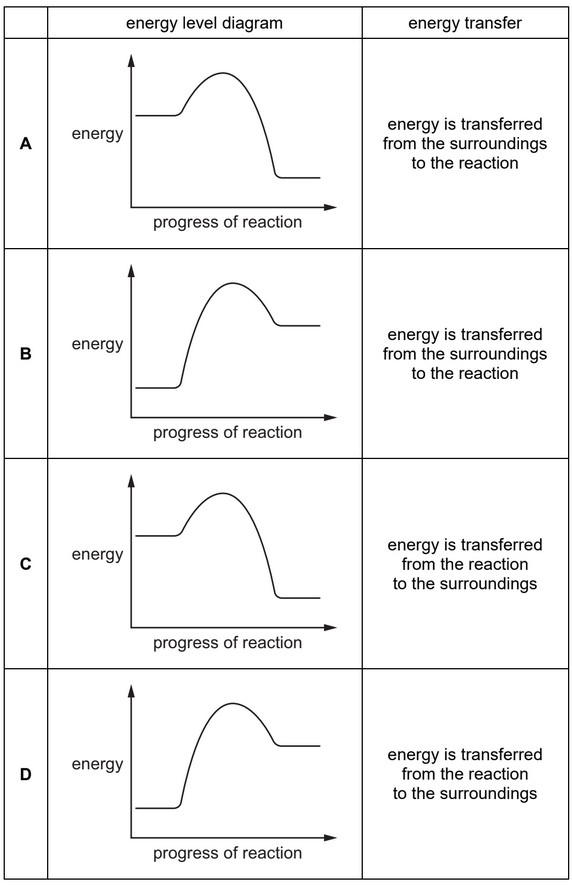
▶️ Answer/Explanation
Ans: B
An endothermic reaction occurs when the energy of the products is higher than that of the reactants, meaning energy is absorbed from the surroundings. This is represented by an upward slope in the energy profile diagram, where the reaction requires net energy input to proceed. Since option B correctly describes this scenario (products at higher energy than reactants), it is the correct answer.
Fuels release heat energy when they burn.
Which substances are used as fuels?
- Argon
- Butane
- Hydrogen
- Methane
A) 1 and 3 only
B) 1, 3 and 4
C) 2, 3 and 4
D) 2 and 4 only
▶️ Answer/Explanation
Ans: C
Fuels are substances that release energy when burned:
1. Argon (1) is a noble gas and does not burn, so it is not a fuel.
2. Butane (2), Hydrogen (3), and Methane (4) are all combustible and used as fuels.
Therefore, the correct combination is 2, 3, and 4 (Option C).
The temperature decreases when aqueous ethanoic acid reacts with solid sodium carbonate to form a salt.
Which type of reaction and energy change occur?
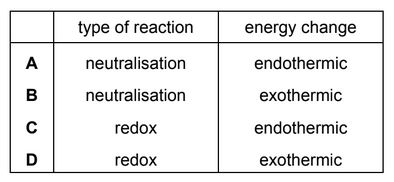
▶️ Answer/Explanation
Ans: A
The reaction between aqueous ethanoic acid (a weak acid) and sodium carbonate (a base) is a neutralization reaction. The temperature decrease indicates the reaction is endothermic, as energy is absorbed from the surroundings to break bonds in the reactants. The products formed are sodium ethanoate, water, and carbon dioxide. Since neutralization reactions typically release heat, this exception (temperature drop) confirms the endothermic nature of this specific acid-carbonate reaction.
Which energy level diagram shows the reaction that will give out the most energy?

▶️ Answer/Explanation
Ans: A
In an exothermic reaction, the energy of the products is lower than the reactants, and the difference (\(\Delta H\)) represents the energy released. Diagram A shows the largest drop in energy, meaning it releases the most energy. The greater the difference between reactant and product energy levels, the more energy is given out.
Dissolving ammonium chloride in water is an endothermic change. Which row shows the energy change and temperature change of the mixture during the dissolving of ammonium chloride?
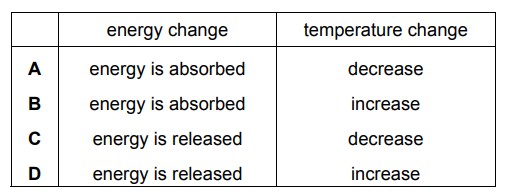
▶️ Answer/Explanation
Ans: A
When ammonium chloride (\( \text{NH}_4\text{Cl} \)) dissolves in water, it absorbs energy from the surroundings (endothermic process). This causes:
- Energy change: The system gains energy (positive change).
- Temperature change: The surroundings lose heat, leading to a decrease in temperature.
Thus, the correct row is A, where energy is absorbed (positive) and temperature decreases.
Which reaction is endothermic?
A \(CaCO_{3}\rightarrow CaO+CO_{2}\)
B \(CaO+2HCl\rightarrow CaCl_{2}+H_{2}O\)
C \(2Ca+O_{2}\rightarrow 2CaO\)
D \(Ca+2HCl\rightarrow CaCl_{2}+H_2\)
▶️ Answer/Explanation
Ans: A
An endothermic reaction absorbs energy from the surroundings. Among the given options:
- Option A (\(CaCO_3 \rightarrow CaO + CO_2\)): This is the thermal decomposition of calcium carbonate, which requires heat to break the bonds in \(CaCO_3\) and form \(CaO\) and \(CO_2\). Hence, it is endothermic.
- Options B, C, and D: These reactions release energy (exothermic).
Thus, the correct answer is A, as it is the only reaction that absorbs energy.
An energy level diagram for a reaction is shown.
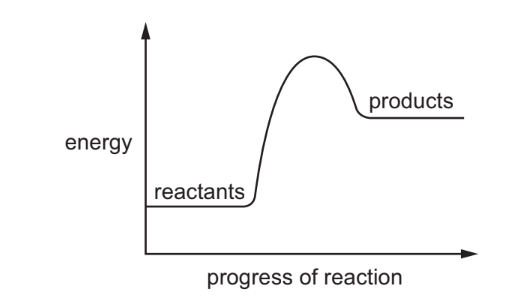
Which statement about the reaction is correct?
A Heat is released.
B It is a combustion reaction.
C It is an endothermic reaction.
D The temperature increases.
▶️ Answer/Explanation
Ans: C
The energy level diagram shows that the products have higher energy than the reactants, indicating an endothermic reaction. In such reactions, energy is absorbed from the surroundings to break bonds, leading to a net increase in system energy. This also means the temperature of the surroundings may decrease, ruling out options A (heat release) and D (temperature increase). Since combustion reactions are exothermic, option B is also incorrect.
Burning fuels is an exothermic reaction.
What is meant by the term exothermic?
- A gas is produced.
- Energy is released.
- Heat is absorbed.
- The mass of the fuel decreases.
▶️ Answer/Explanation
Ans: B
An exothermic reaction is defined by the release of energy (typically as heat or light) to the surroundings. In combustion (burning fuels), the chemical bonds in the fuel break, and new bonds form in the products (e.g., CO₂ and H₂O), releasing energy. While gases may form (A) and mass decreases (D), these are not definitions of exothermicity. Option C describes an endothermic process, making B the correct choice.
A diagram for the energy change during an exothermic reaction is shown.
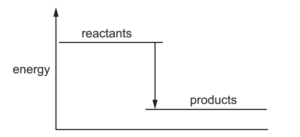
For which reactions would this be an appropriate diagram?
1 CH4 + 2O2 → CO2 + 2H2O
2 2H2 + O2 → 2H2O
3 C + O2 → CO2
A none of them
B 1 and 2 only
C 2 and 3 only
D all of them
▶️ Answer/Explanation
Ans: D
The energy diagram shows an exothermic reaction where products have lower energy than reactants. All three given reactions are combustion reactions, which are inherently exothermic:
- CH4 + 2O2 → CO2 + 2H2O: Releases heat (e.g., burning methane).
- 2H2 + O2 → 2H2O: Highly exothermic (e.g., hydrogen combustion).
- C + O2 → CO2: Releases energy (e.g., burning carbon).
Thus, the diagram applies to all reactions (D).
The energy level diagram for the reaction between sodium hydrogen carbonate and dilute acid is shown.
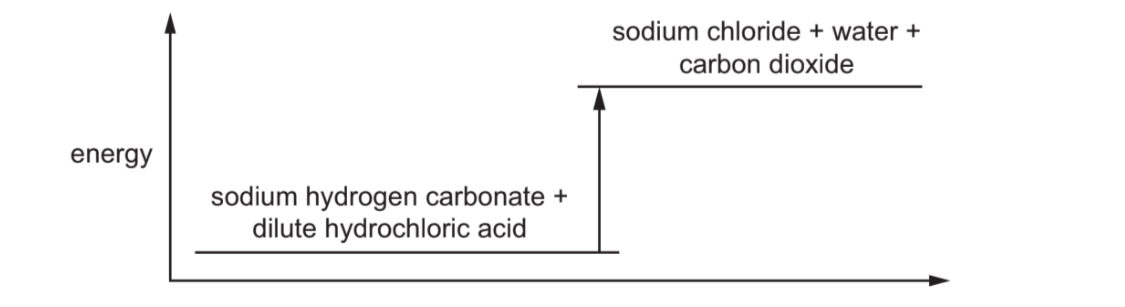
Which row correctly describes the type of reaction and the energy of the reactants and products?
▶️ Answer/Explanation
Ans: A
The energy level diagram shows:
- The products are at a higher energy level than the reactants.
- This indicates an endothermic reaction, where energy is absorbed from the surroundings.
- In such reactions, the temperature of the surroundings typically decreases.
Therefore, the correct description is: endothermic reaction with products at higher energy than reactants (Option A).
The diagrams show the difference in energies of the reactants and products in two types of reaction.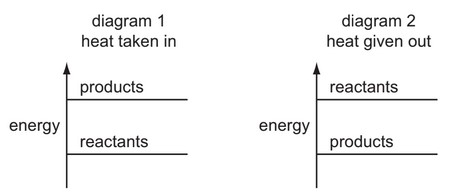
Which diagram and which type of energy change apply to a fuel burning in air?
▶️ Answer/Explanation
Ans: D
Key Points:
- Burning fuel is an exothermic reaction (releases heat).
- In exothermic reactions:
- Reactants have higher energy than products
- Energy is released to surroundings (ΔH is negative)
- Diagram analysis:
- X shows products higher than reactants → endothermic
- Y shows products lower than reactants → exothermic
Therefore, the correct combination is Y (diagram) + exothermic (energy change), which corresponds to option D.
The diagram shows the reaction between zinc oxide and dilute hydrochloric acid.
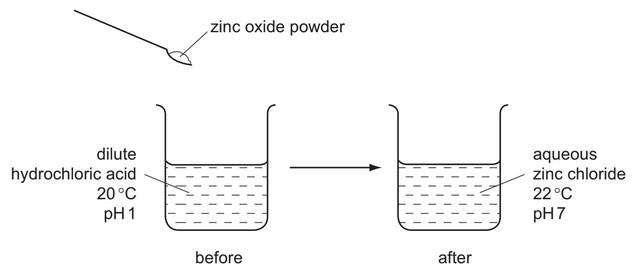
Which terms describe the reaction?
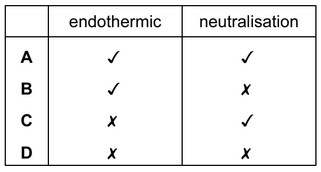
▶️ Answer/Explanation
Ans: C
The reaction between zinc oxide (ZnO) and hydrochloric acid (HCl) can be described as:
- Neutralization: ZnO is a basic oxide reacting with an acid (HCl) to form salt (ZnCl₂) and water (H₂O).
- Exothermic: The reaction releases heat energy, as indicated by the temperature rise.
The chemical equation is:
\[ ZnO(s) + 2HCl(aq) \rightarrow ZnCl_2(aq) + H_2O(l) + \text{heat} \]
Key points:
- It’s not a redox reaction as no oxidation states change.
- Heat release confirms it’s exothermic.
- Acid-base nature confirms neutralization.
Therefore, the correct terms are neutralization and exothermic (Option C).
