Pre AP Chemistry -1.3B Macroscopic Gas Properties and Kinetic Molecular Theory- MCQ Exam Style Questions -New Syllabus 2025-2026
Pre AP Chemistry -1.3B Macroscopic Gas Properties and Kinetic Molecular Theory- MCQ Exam Style Questions – New Syllabus 2025-2026
Pre AP Chemistry -1.3B Macroscopic Gas Properties and Kinetic Molecular Theory- MCQ Exam Style Questions – Pre AP Chemistry – per latest Pre AP Chemistry Syllabus.
The diagram shows a quantity of gas trapped in a cylinder. The piston is pushed in slowly and the gas is compressed. The temperature of the gas does not change.
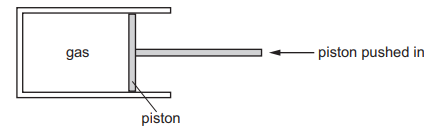
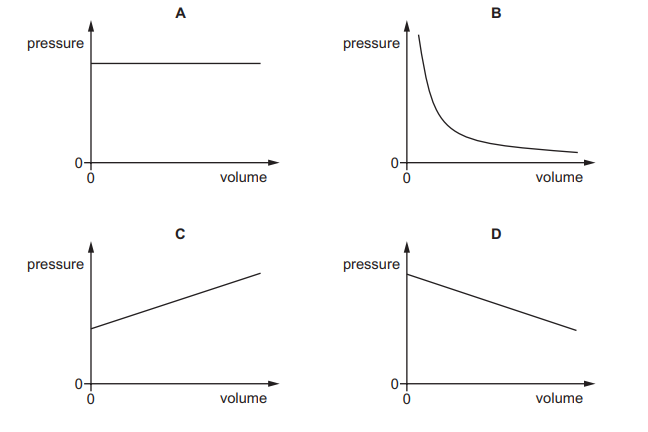
Which graph shows the relationship between the pressure and the volume of the gas?
▶️ Answer/Explanation
Step 1: Identify the law involved
Since temperature remains constant, Boyle’s Law applies.
\( P \propto \frac{1}{V} \)
or
\( PV = \text{constant} \)
Step 2: Relationship between pressure and volume
- As volume decreases, pressure increases.
- The graph of pressure against volume is a curved hyperbola.
Step 3: Choose correct graph
The correct graph is the one showing an inverse (curved) relationship between pressure and volume.
Ans: B
A container is partially filled with hot water, sealed and left to cool.
Which statements are correct?
1 As the temperature decreases, water molecules lose kinetic energy.
2 As the temperature decreases, more water molecules move from vapour to liquid.
3 As the temperature decreases, the vapour pressure of the water decreases.
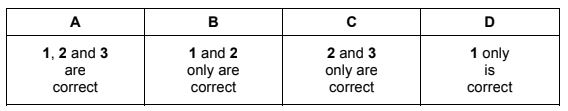
The responses A to D should be selected according to the combinations shown in the diagram.
▶️ Answer/Explanation
Statement 1:
Temperature is proportional to the average kinetic energy of molecules.
When temperature decreases, kinetic energy decreases. ✔
Statement 2:
As the system cools, condensation increases, so more molecules move from vapour to liquid. ✔
Statement 3:
Vapour pressure decreases as temperature decreases. ✔
All three statements are correct.
This corresponds to option A.
Ans: A
What are basic assumptions of the kinetic theory as applied to an ideal gas?
1 Gas particles are in continuous random motion.
2 Gas particles experience no intermolecular forces.
3 The volume of each gas particle is zero.
▶️ Answer/Explanation
Statement 1:
Gas particles are in constant random motion. ✔
Statement 2:
There are no intermolecular forces between ideal gas particles (except during collisions). ✔
Statement 3:
The volume of each particle is assumed negligible (effectively zero compared to the container). ✔
All three statements are correct.
This corresponds to option A.
Answer: A
When molecules of a gas rebound from a wall of a container, the wall experiences a pressure.
What is the cause of this pressure?
A. The change in energy of the molecules.
B. The change in momentum of the molecules.
C. The change in power of the molecules.
D. The change in speed of the molecules.
▶️ Answer/Explanation
Step 1: What happens during collision?
When a gas molecule hits the wall and rebounds, its velocity changes direction.
Step 2: Momentum change
Since momentum depends on velocity, the molecule experiences a change in momentum.
Step 3: Force and pressure
Force is the rate of change of momentum. Many collisions per second create a continuous force on the wall.
Pressure = Force ÷ Area.
Therefore, pressure is caused by the change in momentum of the molecules.
Ans: B
Which statement about the evaporation of a liquid is correct?
A. The least energetic molecules escape from the surface and the temperature of the liquid decreases.
B. The least energetic molecules escape from the surface and the temperature of the liquid increases.
C. The most energetic molecules escape from the surface and the temperature of the liquid decreases.
D. The most energetic molecules escape from the surface and the temperature of the liquid increases.
▶️ Answer/Explanation
Step 1: Understand evaporation
Evaporation occurs when molecules at the surface escape into the vapour phase.
Step 2: Which molecules escape?
Only the most energetic molecules have enough kinetic energy to overcome intermolecular forces.
Step 3: Effect on temperature
When the highest-energy molecules leave, the remaining molecules have lower average kinetic energy.
Since temperature depends on average kinetic energy, the liquid cools.
Therefore:
Most energetic molecules escape and temperature decreases.
Ans: C
During evaporation, molecules escape rapidly from the surface of a liquid.
What happens to the average energy of the molecules of the remaining liquid and what happens to the temperature of the remaining liquid?
| Average energy of remaining molecules | Temperature of remaining liquid | |
|---|---|---|
| A | decreases | decreases |
| B | decreases | increases |
| C | stays the same | decreases |
| D | stays the same | increases |
▶️ Answer/Explanation
Step 1: Which molecules escape?
During evaporation, the most energetic molecules escape from the surface.
Step 2: Effect on remaining liquid
Since the highest-energy molecules leave, the remaining molecules have lower average energy.
Step 3: Link to temperature
Temperature is proportional to average kinetic energy.
If average energy decreases, temperature decreases.
Therefore:
Average energy decreases and temperature decreases.
Ans: A
A swimmer feels cold after leaving warm water on a warm, windy day.
Why does she feel cold even though the air is warm?
A. The less energetic water molecules on her skin escape quickly.
B. The more energetic water molecules on her skin do not escape quickly.
C. The water on her skin does not evaporate quickly enough to keep her warm.
D. The water on her skin evaporates quickly and cools her skin.
▶️ Answer/Explanation
Step 1: Understand evaporation
Evaporation removes the most energetic molecules from the liquid surface.
Step 2: Effect of wind
Wind increases the rate of evaporation by removing water vapour from the surface.
Step 3: Cooling effect
As energetic molecules escape, the average kinetic energy of the remaining water decreases, lowering the skin temperature.
Therefore, she feels cold because evaporation cools her skin.
Ans: D
A fixed mass of gas is trapped in a cylinder with a piston. The volume of the gas is slowly reduced at constant temperature without any particles of gas escaping.
Which statement is correct?
A) The force exerted by the gas on the piston will decrease because the particles move more quickly.
B) The force exerted by the gas on the piston will decrease because the particles move more slowly.
C) The force exerted by the gas on the piston will increase because the particles are hitting the piston harder.
D) The force exerted by the gas on the piston will increase because the particles are hitting the piston more frequently.
▶️ Answer/Explanation
Step 1: Constant temperature
At constant temperature, the average kinetic energy (and speed) of particles remains the same.
Step 2: Volume decreases
When the volume is reduced, particles have less space to move.
Step 3: Effect on collisions
Particles hit the piston more frequently, increasing the force exerted on it.
Pressure increases because collisions per second increase (Boyle’s Law).
Therefore:
The force increases due to more frequent collisions.
Ans: D
What increases when the temperature of a copper block increases?
A the number of copper atoms in the block
B the melting point of the block
C the internal energy of the block
D the density of the block
▶️ Answer/Explanation
Option A: The number of atoms remains constant unless material is added or removed. ✘
Option B: Melting point is a fixed property of the substance and does not increase with temperature. ✘
Option C: When temperature increases, the particles vibrate more. Their kinetic energy increases, so the internal energy increases. ✔
Option D: Density usually decreases slightly due to expansion when heated. ✘
Ans: C
The diagram represents gas particles moving around in a sealed container.
The gas particles collide with the walls of the container.

The temperature of the gas is increased.
What happens to the average speed of the gas particles and what happens to the number of collisions by the gas particles with the walls of the container?
▶️ Answer/Explanation
Step 1: Effect of increasing temperature
Temperature is proportional to the average kinetic energy of gas particles.
When temperature increases, particles move faster.
Step 2: Effect on collisions
If particles move faster, they strike the walls more frequently.
Conclusion:
Average speed increases and number of collisions increases.
Ans: B
In the diagrams, the black circle represents a smoke particle in air.
Which diagram shows a likely path that the particle takes because of Brownian motion?
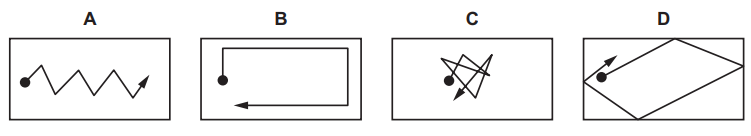
▶️ Answer/Explanation
Brownian motion is the random zig-zag motion of tiny particles suspended in a fluid.
This motion is caused by uneven collisions with fast-moving air molecules.
The correct diagram must show:
- Irregular motion
- Frequent changes in direction
- No smooth or straight path
The diagram that shows this random zig-zag path is C.
Ans: C
Diagram 1 shows apparatus being used to observe smoke particles.
Diagram 2 shows how a smoke particle moves randomly.
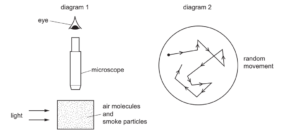
Why do the smoke particles move randomly?
A They are hit by air molecules.
B They are less dense than air.
C They are moved by convection currents.
D They gain energy from the light.
▶️ Answer/Explanation
This motion is an example of Brownian motion.
Air molecules move rapidly and randomly. They collide unevenly with the larger smoke particles.
These random collisions cause the smoke particles to move in an irregular zig-zag path.
Ans: A
