Pre AP Chemistry -3.1A The Mole and Amount of Substance- MCQ Exam Style Questions -New Syllabus 2025-2026
Pre AP Chemistry -3.1A The Mole and Amount of Substance- MCQ Exam Style Questions – New Syllabus 2025-2026
Pre AP Chemistry -3.1A The Mole and Amount of Substance- MCQ Exam Style Questions – Pre AP Chemistry – per latest Pre AP Chemistry Syllabus.
Which statement is the correct definition for molecular formula?
- an atom or group of atoms that determine the chemical properties of a compound
- a structure which shows all the atoms and all the bonds in a compound
- the number and arrangement of different atoms in one gram of a compound
- the number and type of different atoms in one molecule of a compound
▶️ Answer/Explanation
Ans: D
The molecular formula represents the exact number and type of atoms in a single molecule of a compound.
– Option A describes a functional group, not a molecular formula.
– Option B refers to a structural formula.
– Option C is incorrect because molecular formula is not mass-dependent (per gram).
– Only Option D correctly defines molecular formula.
The formula of sodium chlorate(V) is \(NaClO_3\).
What is the relative formula mass of sodium chlorate(V), \(NaClO_3\)?
A) 52.0 B) 74.5 C) 106.5 D) 223.5
▶️ Answer/Explanation
Ans: C
The relative formula mass of \(NaClO_3\) is calculated by summing the atomic masses of all its constituent atoms:
- Sodium (\(Na\)) = 23
- Chlorine (\(Cl\)) = 35.5
- Oxygen (\(O\)) = 16 (and there are 3 oxygen atoms)
Thus, the total mass = \(23 + 35.5 + 3 \times 16 = 23 + 35.5 + 48 = 106.5\).
Therefore, the correct answer is C) 106.5.
What is the relative formula mass of ammonium nitrate, NH4NO3?
A 80 B 108 C 122 D 150
▶️ Answer/Explanation
Ans: A
The relative formula mass (Mr) of NH4NO3 is calculated by summing the atomic masses of all its atoms:
- Nitrogen (N): 14 × 2 = 28 (since there are two nitrogen atoms)
- Hydrogen (H): 1 × 4 = 4 (four hydrogen atoms)
- Oxygen (O): 16 × 3 = 48 (three oxygen atoms)
Total Mr = 28 (N) + 4 (H) + 48 (O) = 80.
Thus, the correct answer is A) 80.
How many atoms of hydrogen are there in a molecule of ethanol, \(C_{2}H_{5}OH\)?
A 1 B 2 C 5 D 6
▶️ Answer/Explanation
Ans: D
The molecular formula of ethanol is \(C_{2}H_{5}OH\).
1. The \(C_{2}H_{5}\) part contains 5 hydrogen atoms.
2. The \(OH\) (hydroxyl group) contributes 1 additional hydrogen atom.
Therefore, the total number of hydrogen atoms is \(5 + 1 = 6\), corresponding to option D.
Iron forms an oxide with the formula Fe2O3.
What is the relative formula mass of this compound?
A) 76
B) 100
C) 136
D) 160
▶️ Answer/Explanation
Ans: D
1. Identify atomic masses:
Iron (Fe) = 56, Oxygen (O) = 16 (from the periodic table).
2. Calculate for Fe2O3:
\(2 \times \text{Fe} = 2 \times 56 = 112\)
\(3 \times \text{O} = 3 \times 16 = 48\)
3. Sum the masses:
\(112 + 48 = 160\).
Thus, the relative formula mass of Fe2O3 is 160.
A compound with the formula \(XF_2\) has a relative formula mass of 78.
What is element X?
- argon
- calcium
- neon
- zirconium
▶️ Answer/Explanation
Ans: B
Given the compound \(XF_2\) with a relative formula mass of 78:
1. Fluorine (\(F\)) has a relative atomic mass of 19.
2. The total mass of two fluorine atoms is \(2 \times 19 = 38\).
3. Subtract the mass of fluorine from the total mass to find \(X\): \(78 – 38 = 40\).
4. The element with a relative atomic mass of 40 is calcium (\(Ca\)).
Thus, the correct answer is B (calcium).
A compound has the formula \(CH_3CO_2H\).
How should the relative molecular mass, \(M_r\), of this compound be calculated?
A) 12 + 1 + 16
B) 3(12 + 1) + 2(12 + 16) + 1
C) (4 × 12) + (2 × 1) + 16
D) (2 × 12) + (4 × 1) + (2 × 16)
▶️ Answer/Explanation
Ans: D
The relative molecular mass (\(M_r\)) of \(CH_3CO_2H\) is calculated by summing the atomic masses of all atoms in the formula:
- Carbon (\(C\)) = 12 (2 atoms)
- Hydrogen (\(H\)) = 1 (4 atoms)
- Oxygen (\(O\)) = 16 (2 atoms)
Thus, the calculation is: \((2 \times 12) + (4 \times 1) + (2 \times 16)\).
Breaking it down:
- From \(CH_3\): 1 C and 3 H
- From \(CO_2H\): 1 C, 2 O, and 1 H
- Total: 2 C, 4 H, and 2 O.
Therefore, the correct calculation is D) (2 × 12) + (4 × 1) + (2 × 16).
What is the relative formula mass of magnesium bromide?
A) 47
B) 82
C) 104
D) 184
▶️ Answer/Explanation
Ans: D
First, we need to know:
- Magnesium (Mg) has atomic mass ≈ 24
- Bromine (Br) has atomic mass ≈ 80
The formula for magnesium bromide is MgBr₂ (magnesium has 2+ charge, bromide has 1- charge).
Calculating relative formula mass:
\[ \text{MgBr}_2 = 24 + (2 \times 80) = 24 + 160 = 184 \]
Therefore, the correct answer is 184.
Common mistake would be forgetting there are two bromine atoms in the formula.
What is the relative molecular mass, Mr, of sulfuric acid, H2SO4?
A) 81
B) 82
C) 97
D) 98
▶️ Answer/Explanation
Ans: D
To calculate the relative molecular mass (Mr) of sulfuric acid (H2SO4):
- Hydrogen (H): 2 atoms × 1 = 2
- Sulfur (S): 1 atom × 32 = 32
- Oxygen (O): 4 atoms × 16 = 64
Total Mr = 2 + 32 + 64 = 98
Remember to use the relative atomic masses from the periodic table:
- H = 1
- S = 32
- O = 16
The correct answer is therefore D) 98.
A compound, T, has the formula \(CH_3Cl\). Three statements about this compound are listed.
- A molecule of the compound contains five atoms.
- A molecule of the compound contains five different elements.
- The relative molecular mass of the compound is 50.5.
Which statements are correct?
- 1, 2 and 3
- 1 and 2 only
- 1 and 3 only
- 2 and 3 only
▶️ Answer/Explanation
Ans: C
Analyzing the given statements for \(CH_3Cl\):
1. Correct: The molecule has 1 C + 3 H + 1 Cl = 5 atoms.
2. Incorrect: It contains only 3 elements (C, H, Cl), not five.
3. Correct: Relative molecular mass = 12 (C) + 3×1 (H) + 35.5 (Cl) = 50.5.
Thus, only statements 1 and 3 are correct.
What is the definition of relative molecular mass, \(M_r\)?
A It is the average mass of the isotopes in a compound.
B It is the sum of the atomic numbers in a compound.
C It is the sum of the relative atomic masses in a compound.
D It is the total number of atoms in a compound.
▶️ Answer/Explanation
Ans: C
The relative molecular mass (\(M_r\)) is defined as:
- Option A is incorrect because \(M_r\) is not an average of isotopes but a sum of atomic masses.
- Option B is incorrect because atomic numbers (proton counts) are irrelevant to mass calculations.
- Option C is correct: \(M_r\) is the sum of the relative atomic masses of all atoms in a molecule (e.g., \(M_r\) of H2O = 1 + 1 + 16 = 18).
- Option D is incorrect because \(M_r\) represents mass, not atom count.
Thus, the accurate definition is given in C.
The relative atomic mass, \( A_r \), of an element is the average mass of the isotopes of that element compared to another particle. Which particle is used for this comparison?
A) a proton
B) an atom of \( ^{12}C \)
C) an atom of \( ^{40}Ca \)
D) an atom of \( ^1H \)
▶️ Answer/Explanation
Ans: B
1. The relative atomic mass (\( A_r \)) is defined as the average mass of an element’s isotopes compared to 1/12th the mass of a \( ^{12}C \) atom.
2. This standard was established by IUPAC for consistency in atomic mass measurements.
3. \( ^{12}C \) is used because it is a stable isotope with a well-defined mass, making calculations precise.
4. Thus, the correct comparison particle is an atom of \( ^{12}C \) (Option B).
The relative atomic mass of chlorine is 35.5.
When calculating relative atomic mass, which particle is the mass of a chlorine atom compared to?
A) a neutron
B) a proton
C) an atom of carbon-12
D) an atom of hydrogen-1
▶️ Answer/Explanation
Ans: C
The relative atomic mass (Ar) of an element is defined as the average mass of its atoms compared to 1/12th the mass of a carbon-12 atom. This is because:
- The carbon-12 isotope (12C) is assigned a mass of exactly 12 atomic mass units (amu) by international agreement.
- Chlorine’s relative atomic mass (35.5) means a chlorine atom is, on average, 35.5/12 times heavier than 1/12th of a carbon-12 atom.
Why other options are incorrect:
- A (Neutron) and B (Proton): Relative atomic mass is not compared to individual nucleons.
- D (Hydrogen-1): Early definitions used hydrogen, but carbon-12 is now the standard for greater precision.
What is the relative formula mass of magnesium nitrate, \(Mg(NO_3)_2\)?
A 74 B 86 C 134 D 148
▶️ Answer/Explanation
Ans: D
To calculate the relative formula mass of \(Mg(NO_3)_2\):
1. Magnesium (Mg): 1 atom × 24 = 24
2. Nitrogen (N): 2 atoms × 14 = 28 (since there are 2 nitrate groups, each with 1 nitrogen)
3. Oxygen (O): 6 atoms × 16 = 96 (each nitrate group has 3 oxygens, and there are 2 groups)
Total: 24 (Mg) + 28 (N) + 96 (O) = 148
Thus, the correct answer is D (148).
A compound has the formula \(XF_2\) and has a relative mass of 70.
What is element X?
A) gallium
B) germanium
C) sulfur
D) ytterbium
▶️ Answer/Explanation
Ans: C
1. Given data:
Fluorine (F) has a relative atomic mass of 19.
The compound \(XF_2\) has a total relative mass of 70.
2. Set up the equation:
\(X + 2 \times 19 = 70\)
\(X + 38 = 70\)
3. Solve for X:
\(X = 70 – 38 = 32\)
4. Identify the element:
The element with a relative atomic mass of 32 is sulfur (S).
Thus, element X is sulfur (option C).
The compound magnesium nitrate has the formula \(Mg(NO_{3})_{2}\). What is the relative formula mass of magnesium nitrate?
A. 86 B. 134 C. 148 D. 172
▶️ Answer/Explanation
Ans: C
To calculate the relative formula mass of \(Mg(NO_3)_2\):
1. Magnesium (Mg): Atomic mass = 24
2. Nitrate (\(NO_3\)): Each nitrate group has 1 nitrogen (N = 14) and 3 oxygens (O = 16 × 3 = 48).
3. Since there are 2 nitrate groups, total mass = 2 × (14 + 48) = 2 × 62 = 124.
4. Total mass = Mg + 2 nitrate groups = 24 + 124 = 148.
Thus, the correct answer is C (148).
The relative formula mass, Mr, of calcium carbonate, CaCO3, is 100.
What is the mass of carbon present in 100 g of calcium carbonate?
A 12 g B 36 g C 40 g D 60 g
▶️ Answer/Explanation
Ans: A
1. The formula mass of CaCO3 is 100, where:
- Calcium (Ca) contributes 40
- Carbon (C) contributes 12
- Oxygen (O3) contributes 48 (3 × 16)
2. In 100 g of CaCO3, the mass ratio of carbon is:
\[ \frac{12}{100} \times 100\, \text{g} = 12\, \text{g} \]
Thus, the mass of carbon present is 12 g (Option A).
Three chemical reactions are shown.
- catalytic addition of steam to ethene
- combustion of ethanol
- fermentation of glucose
In which of the reactions does the relative molecular mass of the carbon-containing compound decrease?
A) 1 and 2 only B) 1 and 3 only C) 2 and 3 only D) 1, 2 and 3
▶️ Answer/Explanation
Ans: C
We analyze each reaction to determine whether the relative molecular mass (\(M_r\)) of the carbon-containing compound decreases:
- Catalytic addition of steam to ethene:
\(C_2H_4 + H_2O \rightarrow C_2H_5OH\)
\(M_r\) of \(C_2H_4\) = 28 g/mol → \(M_r\) of \(C_2H_5OH\) = 46 g/mol.
Result: \(M_r\) increases (not a decrease). - Combustion of ethanol:
\(C_2H_5OH + 3O_2 \rightarrow 2CO_2 + 3H_2O\)
\(M_r\) of \(C_2H_5OH\) = 46 g/mol → \(M_r\) of \(CO_2\) = 44 g/mol.
Result: \(M_r\) decreases. - Fermentation of glucose:
\(C_6H_{12}O_6 \rightarrow 2C_2H_5OH + 2CO_2\)
\(M_r\) of \(C_6H_{12}O_6\) = 180 g/mol → \(M_r\) of \(C_2H_5OH\) = 46 g/mol.
Result: \(M_r\) decreases.
Only reactions 2 and 3 show a decrease in \(M_r\) for the carbon-containing compound. Thus, the correct answer is C) 2 and 3 only.
What is the definition of relative atomic mass, Ar?
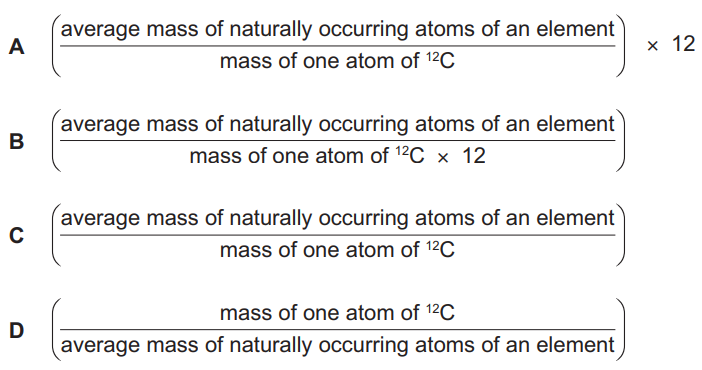
▶️ Answer/Explanation
Ans: A
The relative atomic mass (Ar) is defined as:
- The weighted average mass of an atom of an element
- Compared to 1/12th the mass of a carbon-12 atom
- Taking into account the natural abundance of its isotopes
This matches option A in the image, which correctly describes this definition. The other options are incorrect because:
- B refers to molecular mass rather than atomic mass
- C describes mass number (protons + neutrons)
- D incorrectly compares to hydrogen rather than carbon-12
What is the relative formula mass, Mr, of CaCO3?
A 50 B 68 C 100 D 204
▶️ Answer/Explanation
Ans: C
To calculate the relative formula mass (Mr) of calcium carbonate (CaCO3):
- Calcium (Ca): 1 atom × 40 = 40
- Carbon (C): 1 atom × 12 = 12
- Oxygen (O): 3 atoms × 16 = 48
Total Mr = 40 (Ca) + 12 (C) + 48 (O) = 100.
Therefore, the correct answer is C) 100.
In athletics, banned drugs such as nandrolone have been taken illegally to improve performance. Nandrolone has the molecular formula C18H26O2.
What is the relative molecular mass, Mr, of nandrolone?
(Relative atomic mass: H = 1; C = 12; O = 16)
A) 46 B) 150 C) 274 D) 306
▶️ Answer/Explanation
Ans: C
Step 1: Calculate the contribution from carbon atoms
There are 18 carbon atoms, each with a relative atomic mass of 12.
\(18 \times 12 = 216\)
Step 2: Calculate the contribution from hydrogen atoms
There are 26 hydrogen atoms, each with a relative atomic mass of 1.
\(26 \times 1 = 26\)
Step 3: Calculate the contribution from oxygen atoms
There are 2 oxygen atoms, each with a relative atomic mass of 16.
\(2 \times 16 = 32\)
Step 4: Sum all contributions
\(216 (C) + 26 (H) + 32 (O) = 274\)
Therefore, the relative molecular mass (Mr) of nandrolone is 274.
Cesium chloride and rubidium bromide are halide compounds of Group I elements.
Cesium chloride has the formula ___ 1 ___ , a relative formula mass ___ 2 ___ that of rubidium bromide and bonds that are ___ 3 ___ .
Which words correctly complete gaps 1, 2 and 3?
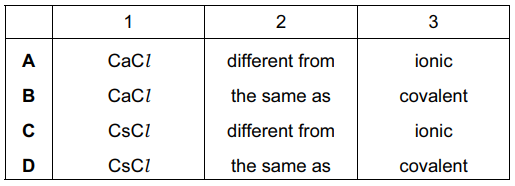
▶️ Answer/Explanation
Ans: C
1. Formula (Gap 1): Cesium chloride is CsCl because Cs+ and Cl– form a 1:1 ionic compound.
2. Relative Formula Mass (Gap 2):
– CsCl: \( 132.9\ (Cs) + 35.45\ (Cl) = 168.35\ \text{g/mol} \)
– RbBr: \( 85.47\ (Rb) + 79.9\ (Br) = 165.37\ \text{g/mol} \)
Since \( 168.35 > 165.37 \), CsCl has a greater formula mass than RbBr.
3. Bond Type (Gap 3): Both compounds form ionic bonds due to electron transfer between Group I metals and halogens.
Thus, the correct option is C (CsCl, greater, ionic).
Which relative molecular mass, Mr, is not correct for the molecule given?
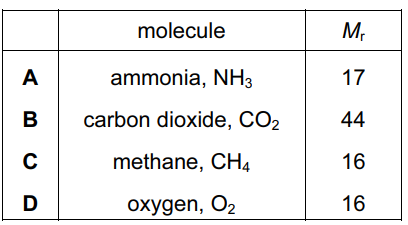
▶️ Answer/Explanation
Ans: D
The relative molecular mass (Mr) is calculated by summing the relative atomic masses of all atoms in the molecule:
| Molecule | Calculation | Correct Mr |
|---|---|---|
| NH3 | 14 + (3 × 1) = 17 | 17 (matches) |
| CO2 | 12 + (2 × 16) = 44 | 44 (matches) |
| H2O | (2 × 1) + 16 = 18 | 18 (matches) |
| O2 | 2 × 16 = 32 | 16 (incorrect) |
Key Points:
- O2 consists of two oxygen atoms (each with Ar = 16), so its Mr should be 32.
- The table incorrectly shows O2 as 16, which is the Ar of a single oxygen atom.
- All other molecules (NH3, CO2, H2O) have correct Mr values.
What is the relative molecular mass, \(M_r\), of sulfur dioxide?
A) 24
B) 32
C) 48
D) 64
▶️ Answer/Explanation
Ans: D
The relative molecular mass (\(M_r\)) of sulfur dioxide (\(SO_2\)) is calculated as follows:
– Sulfur (\(S\)) has an atomic mass of 32.
– Oxygen (\(O\)) has an atomic mass of 16, and there are two oxygen atoms.
– Therefore, \(M_r = 32 + (2 \times 16) = 64\).
Thus, the correct answer is D (64).
The relative atomic mass, \(A_r\), of an element is determined by comparing the mass of one atom of the element with the mass of one atom of element Q.
What is Q?
- carbon
- chlorine
- hydrogen
- oxygen
▶️ Answer/Explanation
Ans: A (carbon)
The relative atomic mass (\(A_r\)) is defined as the average mass of an atom of an element compared to \(\frac{1}{12}\) the mass of a carbon-12 atom.
Key points:
- Carbon-12 (\(^{12}C\)) is the standard reference for atomic masses.
- 1 atomic mass unit (u) is defined as \(\frac{1}{12}\) the mass of a carbon-12 atom.
- Thus, element Q must be carbon.
2.56 g of a metal oxide, \(MO_2\), is reduced to 1.92 g of the metal, M.
What is the relative atomic mass of M?
A) 48
B) 96
C) 128
D) 192
▶️ Answer/Explanation
Ans: B
1. Mass of oxygen in \(MO_2\): \[ 2.56\, \text{g} – 1.92\, \text{g} = 0.64\, \text{g} \] 2. Moles of oxygen: \[ \frac{0.64\, \text{g}}{16\, \text{g/mol}} = 0.04\, \text{mol} \] 3. Since \(MO_2\) contains 2 oxygen atoms, moles of \(MO_2\): \[ \frac{0.04\, \text{mol}}{2} = 0.02\, \text{mol} \] 4. Molar mass of M: \[ \frac{1.92\, \text{g}}{0.02\, \text{mol}} = 96\, \text{g/mol} \] Thus, the relative atomic mass of M is 96 (Option B).
The relative atomic mass of chlorine is 35.5.
When calculating relative atomic mass, which particle is the mass of a chlorine atom compared to?
A) a neutron
B) a proton
C) an atom of carbon-12
D) an atom of hydrogen-1
▶️ Answer/Explanation
Ans: C
1. Relative atomic mass is defined as the average mass of an atom compared to 1/12th the mass of a carbon-12 atom.
2. Carbon-12 (\(^{12}C\)) is the standard reference in atomic mass units (amu), where its mass is exactly 12.
3. Chlorine’s relative atomic mass (35.5) means it is 35.5 times heavier than 1/12th of a carbon-12 atom.
4. Options A, B, and D are incorrect because they do not represent the standard reference used in atomic mass calculations.
An oxide of nitrogen has the following composition by mass: N, 30.4%; O, 69.6%.
It has a relative molecular mass of 92.
What is the molecular formula of the oxide of nitrogen?
A) NO
B) NO2
C) NO4
D) N2O4
▶️ Answer/Explanation
Ans: D
1. Calculate moles of each element:
– Nitrogen: \( \frac{30.4}{14} = 2.17 \) mol
– Oxygen: \( \frac{69.6}{16} = 4.35 \) mol
2. Determine simplest ratio:
– Divide by smallest (2.17): N = 1, O = 2
– Empirical formula: NO2
3. Find molecular formula:
– Empirical mass = 14 + (16 × 2) = 46
– \( \frac{92}{46} = 2 \) → Molecular formula = N2O4
Thus, the correct answer is D (N2O4).
A compound, X, contains 40.0% carbon, 6.7% hydrogen and 53.3% oxygen by mass. The relative molecular mass, \(M_r\), of X is 60. What is the molecular formula of X?
A) \(CH_2O\)
B) \(CH_4O\)
C) \(C_2H_4O\)
D) \(C_2H_4O_2\)
▶️ Answer/Explanation
Ans: D
Step 1: Determine the empirical formula
– Assume 100 g of the compound: \[ \text{Carbon (C)} = 40.0\,g \quad (\frac{40.0}{12} = 3.33\,mol) \] \[ \text{Hydrogen (H)} = 6.7\,g \quad (\frac{6.7}{1} = 6.7\,mol) \] \[ \text{Oxygen (O)} = 53.3\,g \quad (\frac{53.3}{16} = 3.33\,mol) \] – Divide by the smallest number of moles (3.33): \[ \text{C} : \text{H} : \text{O} = 1 : 2 : 1 \] – Empirical formula = \(CH_2O\) (Mr = 30).
Step 2: Find the molecular formula
– Given \(M_r = 60\), the ratio is: \[ \frac{60}{30} = 2 \] – Thus, the molecular formula = \((CH_2O)_2 = C_2H_4O_2\).
Therefore, the correct answer is D (\(C_2H_4O_2\)).
