Pre AP Chemistry -3.1B Ideal Gas Law and Gas Particles- MCQ Exam Style Questions -New Syllabus 2025-2026
Pre AP Chemistry -Link- MCQ Exam Style Questions – New Syllabus 2025-2026
Pre AP Chemistry -Link- MCQ Exam Style Questions – Pre AP Chemistry – per latest Pre AP Chemistry Syllabus.
Question
\(10cm^{3}\) of ethane is burned in \(45cm_{3}\) of oxygen at a pressure of 101kPa and a temperature of 200 °C. Complete combustion takes place.
What is the total volume of gas present when the reaction is complete, measured under the same conditions?
A \(30cm^{3}\) B \(50cm^{3}\) C \(55cm^{3}\) D \(60cm^{3}\)
▶️Answer/Explanation
Ans:D
Question:
1.8 g of water, heated to 227 °C in a sealed container, turns to steam with a pressure of 200 kPa.
What is the approximate volume of the container?
A \(9\times10^{-4}m^{3}\) B \(2\times10^{3}m^{3}\) C \(2m^{3}\) D \(8\times10^{7}m^{3}\)
▶️Answer/Explanation
Ans:C
Question
The gas laws can be summarised in the ideal gas equation.
pV = nRT
0.960 g of oxygen gas is contained in a vessel of volume 7.00 × 10–3 m3 at a temperature of 30 °C.
Assume that the gas behaves as an ideal gas.
What is the pressure in the vessel?
A 1.07 kPa B 2.14 kPa C 10.8 kPa D 21.6 kPa
Answer/Explanation
Answer:
C
Question
A fluorescent light tube has an internal volume of 400 \(cm^3\) and an internal pressure of 200 kPa. It is filled with 0.03 moles of an ideal gas.
What is the temperature of the gas inside the fluorescent light tube?
A 3.21 × \(10^{–1}\)K
B 3.21 × \(10^2\)K
C 3.21 × \(10^5\)K
D 3.21 × \(10^8\)K
Answer/Explanation
Ans: B
Question
Use of the Data Booklet is relevant to this question. The gas laws can be summarised in the ideal gas equation below.
pV = nRT
0.96 g of oxygen gas is contained in a glass vessel of volume \( 7.0 × 10^{–3}m^{3}\) at a temperature of 30°C. Assume the gas behaves as an ideal gas.
What is the pressure in the vessel?
A 1.1kPa B 2.1kPa C 10.8kPa D 21.6kPa
Answer/Explanation
Ans:C
Question .
The gas laws can be summarised in the ideal gas equation.
pV = nRT
where each symbol has its usual meaning.
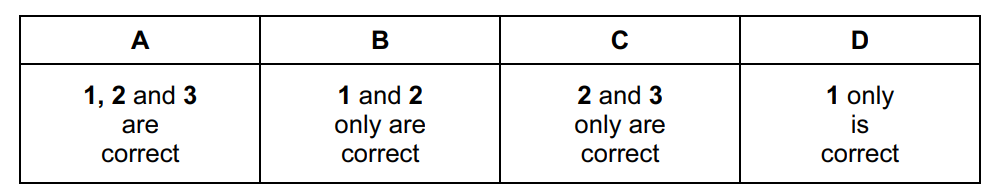
Which statements are correct?
- One mole of an ideal gas occupies the same volume under the same conditions of temperature and pressure.
- The density of an ideal gas at constant pressure is inversely proportional to the temperature, T.
- The volume of a given mass of an ideal gas is doubled if its temperature is raised from 25 °C to 50°C at constant pressure.
Answer/Explanation
Ans:
B
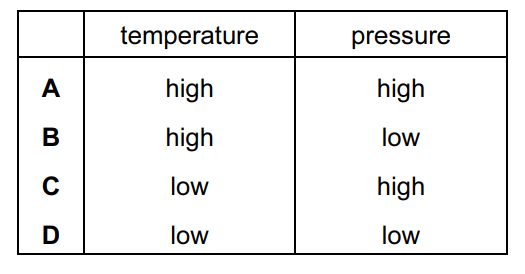
Question
Under which set of conditions is a gas most likely to behave ideally?
Answer/Explanation
Ans:
B
Question
Use of the Data Booklet is relevant to this question.
The gas laws can be summarised in the ideal gas equation.
pV = nRT
0.56g of ethene gas is contained in a vessel at a pressure of 102kPa and a temperature of 30°C.
What is the volume of the vessel?
A 49 cm3 B 494 cm3 C 48 900 cm3 D 494 000 cm3
Answer/Explanation
Ans:
B
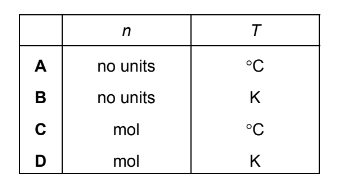
Question
In the ideal gas equation, pV = nRT, what are the units of n and T?
Answer/Explanation
Answer: D
