Question
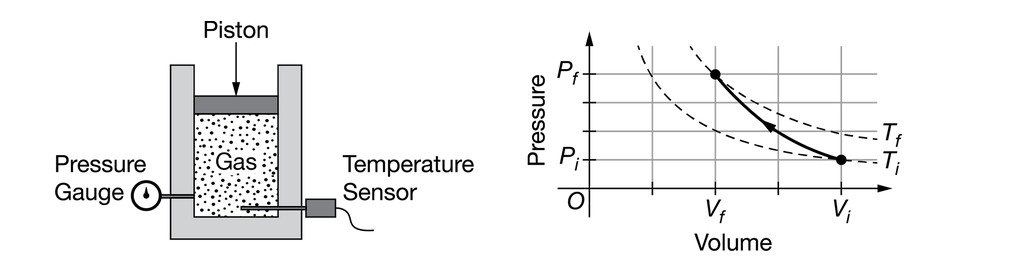
A gas is in a sealed cylinder fitted with a movable piston, as shown in the figure, so that none of the gas escapes. The cylinder and piston are made of an insulating material. The cylinder is fitted with pressure and temperature sensors, and the volume of the confined gas can be measured from markings on the side of the cylinder. The gas is initially in a state with pressure \(P_i\), temperature \(T_i\), and volume \(V_i\). The piston is slowly compressed and data are recorded until the gas reaches a final state with pressure \(P_f\), temperature \(T_f\), and volume \(V_f\). A graph of the pressure as a function of volume is shown, with dotted lines indicating isotherms.
Which of the following describes the energy transfer process that the insulation prevents?
A Friction between gas molecules moving along the inner surface of the cylinder transfers energy through the cylinder to the air molecules that move along the outer surface of the cylinder
B Electromagnetic waves produced by the motion of the electric charges in the gas molecules are absorbed by air molecules outside the cylinder
C Convection currents in the gas molecules induce similar currents in the cylinder and the air around the cylinder
D Collisions of gas molecules with the inner surface of the cylinder cause disturbances of molecules of the insulating material that travel through the cylinder, transferring energy to air molecules that hit the outer surface of the cylinder
▶️Answer/Explanation
Ans:D
The insulation prevents energy loss by conduction.
Question
A student is sitting on the ground a few meters away from a campfire on a cool, windless night. The student feels noticeably warm on the side facing toward the fire, and noticeably cool on the side facing away from the fire. Which of the following models describes the primary method by which the side of the student facing toward the fire is warmed?
A The burning wood heats the surrounding air. Some thermal energy is conducted through the air to the student.
B The burning wood heats the surrounding air. As the heated air expands, it comes in contact with the student.
C The fire emits radiation in the form of charged particles. Some of those particles strike the student.
D The fire emits electromagnetic radiation. Some of the radiation is absorbed by the student and is converted to thermal energy.
▶️Answer/Explanation
Ans:D
Energy is transferred by radiation and absorbed by the student.
Question
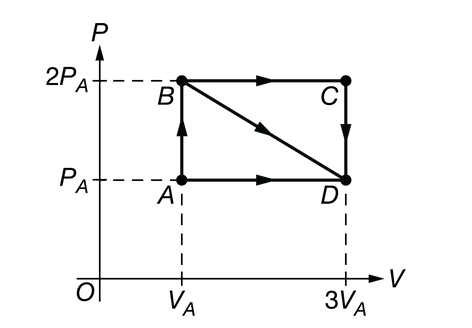
A sample of an ideal gas is in a sealed cylinder with a movable piston and is initially in state A, as shown in the graph of pressure P as a function of volume V. The gas’s temperature in state A is 300K. The gas can be taken from state A to state D via three separate paths, AD, ABD, or ABCD.
In which process is energy transferred from the gas to the environment by heating only?
A \(AB\)
B \(BC\)
C \(BD\)
D \(CD\)
▶️Answer/Explanation
Ans:D
The only transfer of energy is by heating because the area under the curve is zero, and the energy is being transferred from the gas to the environment, since the temperature of the gas is decreasing.
Question
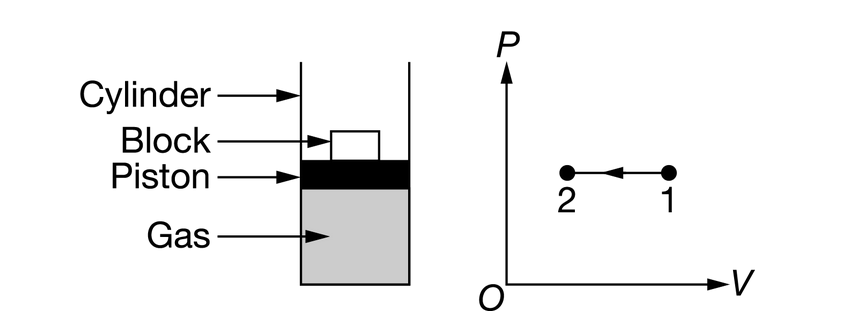
The figure shows a cylinder that has a movable piston and contains an ideal gas initially in state 1 at room temperature. The cylinder is sealed. Blocks of known mass can be added to or removed from the top of the piston. The gas is taken through the process represented by the graph of pressure P as a function of volume V .
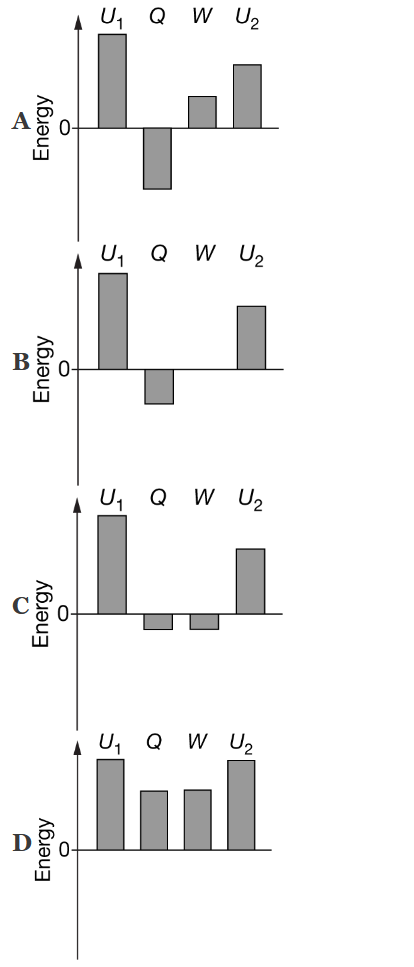
Which of the following energy bar charts could represent the process as the gas is taken from state 1 to state 2, where \(U_1\) represents the initial internal energy of the N molecules of gas, Q represents the energy transferred to the gas by heating, W represents the work done on the gas, and \(U_2\) represents the final internal energy of the gas?
▶️Answer/Explanation
Ans:A
During the process, the gas loses thermal energy, and positive work is done on the gas. By the first law of thermodynamics, the sum of the change in thermal energy and the work done on the gas is equal to the change in the internal energy.
Question
A sample of an ideal gas is contained in a cylinder with a moveable piston. The gas expands, doing work W on the piston and absorbing thermal energy Q from its surroundings. Which of the following identifies and justifies the correct form of the first law of thermodynamics during this process?
A \(ΔU=Q−W\) , because thermal energy is transferred to the gas and work is done by the gas.
B \(ΔU=Q+W\) , because thermal energy is transferred to the gas and work is done on the gas.
C \(ΔU=−Q−W\) , because thermal energy is transferred to the surroundings and work is done by the gas.
D \(ΔU=−Q+W\) , because thermal energy is transferred to the surroundings and work is done on the gas.
▶️Answer/Explanation
Ans:A
Energy is transferred to the gas by heating, which acts to increase the internal energy, and work is done by the gas, which acts to decrease the internal energy.
