7.5 g of nitrogen monoxide reacts with 7.0 g of carbon monoxide on the surface of the catalytic converter in the exhaust system of a car. What is the total volume of the product gases measured at room conditions?
▶️ Answer/Explanation
Ans: C
The catalytic reaction in a car’s exhaust system is: \[ 2NO(g) + 2CO(g) \rightarrow N_2(g) + 2CO_2(g) \] Step 1: Calculate moles of reactants
– Moles of NO = \(\frac{7.5\, \text{g}}{30\, \text{g/mol}} = 0.25\, \text{mol}\)
– Moles of CO = \(\frac{7.0\, \text{g}}{28\, \text{g/mol}} = 0.25\, \text{mol}\)
The reactants are in a 1:1 stoichiometric ratio (as per the equation), so neither is limiting.
Step 2: Determine moles of products
– Moles of N₂ produced = \(\frac{0.25\, \text{mol NO}}{2} = 0.125\, \text{mol}\)
– Moles of CO₂ produced = \(0.25\, \text{mol CO} = 0.25\, \text{mol}\)
Total moles of gas produced = 0.125 + 0.25 = 0.375 mol
Step 3: Calculate volume at room conditions
At room temperature and pressure (RTP), 1 mole of gas occupies 24 dm³.
Thus, total volume = \(0.375\, \text{mol} \times 24\, \text{dm³/mol} = 9.0\, \text{dm³}\).
Therefore, the correct answer is C (9.0 dm³).
What contains the greatest number of the named particles?
A. 6.0 dm³ of argon atoms at room conditions
B. 6.0 g of carbon dioxide molecules
C. 6.0 g of magnesium atoms
D. 6.0 g of water molecules
▶️ Answer/Explanation
Ans: D
To determine the greatest number of particles, we compare the number of moles for each option. Option D (water molecules) has the highest number of moles because water (\(H_2O\)) has a low molar mass (~18 g/mol), meaning 6.0 g contains \(\frac{6.0}{18} = 0.33\) moles. In contrast, CO₂ (44 g/mol) has 0.14 moles, Mg (24 g/mol) has 0.25 moles, and 6.0 dm³ of Ar at room conditions is ~0.25 moles. Thus, water has the most particles.
Glauber’s salt consists of crystals of hydrated sodium sulfate, Na₂SO₄•xH₂O, which can be used for the manufacture of detergents. When a sample of Glauber’s salt was heated, 1.91 g of water was removed leaving 1.51 g of anhydrous Na₂SO₄. What is the value of x in Na₂SO₄•xH₂O?
▶️ Answer/Explanation
Ans: C
1. Calculate the moles of anhydrous Na₂SO₄: \[ n = \frac{1.51 \text{ g}}{142.04 \text{ g/mol}} = 0.0106 \text{ mol} \]
2. Calculate the moles of water removed: \[ n = \frac{1.91 \text{ g}}{18.015 \text{ g/mol}} = 0.106 \text{ mol} \]
3. Determine the ratio of H₂O to Na₂SO₄: \[ x = \frac{0.106}{0.0106} = 10 \]
Thus, the formula is Na₂SO₄•10H₂O, making C (10) the correct answer.
The diagram shows the mass spectrum of a sample of chlorine. Peaks V, W, X, Y and Z are labelled.
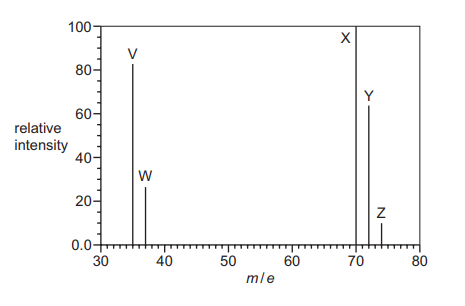
Which statements about this spectrum are correct?
1 The relative atomic mass of chlorine can be calculated from the abundances and m/ e values of 2 of the 5 peaks.
2 37.0 g of the species responsible for peak Z contains \(3.011 \times 10^{23}\) molecules.
3 The relative molecular mass of chlorine can be calculated from the abundances and m/ e values of peaks X, Y and Z.
▶️ Answer/Explanation
Ans: A
Statement 1: Correct. The relative atomic mass of chlorine is calculated using the isotopic peaks (V and W for \(^{35}\text{Cl}\) and \(^{37}\text{Cl}\)) and their abundances. Only these two peaks are needed.
Statement 2: Correct. Peak Z corresponds to \(\text{Cl}_2\) with \(m/e = 74\) (\(^{37}\text{Cl}-^{37}\text{Cl}\)). 37.0 g is 0.5 moles, containing \(0.5 \times 6.022 \times 10^{23} = 3.011 \times 10^{23}\) molecules.
Statement 3: Correct. The relative molecular mass of \(\text{Cl}_2\) is derived from peaks X (\(^{35}\text{Cl}-^{35}\text{Cl}\), \(m/e = 70\)), Y (\(^{35}\text{Cl}-^{37}\text{Cl}\), \(m/e = 72\)), and Z (\(^{37}\text{Cl}-^{37}\text{Cl}\), \(m/e = 74\)), weighted by their abundances.
Thus, all three statements (1, 2, and 3) are correct, making option A the answer.
