Question:
Where in the Periodic Table is the element that has an outer electron shell arrangement of \(4s^{2}p^{3}\)
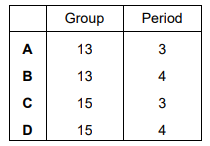
▶️Answer/Explanation
Ans:D
Question:
The table shows the numbers of protons, neutrons and electrons in four different particles, W, X, Y, and Z.
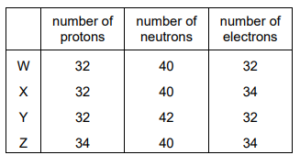
Which pair represents the atoms of two isotopes of the same element?
▶️Answer/Explanation
Ans:A
Question
A single ${ }^{32} \mathrm{P}$ nucleus can be produced when a single ${ }^{32} \mathrm{~S}$ nucleus joins with particle $\mathrm{X}$. In the process a proton is emitted.
What is particle $X$ ?
A a deuteron, ${ }_1^2 \mathrm{H}^{+}$
B an electron
C a neutron
D a proton
▶️Answer/Explanation
Ans:C
Question
In which pair does each species have the same number of unpaired electrons?
A $\mathrm{A} l$ and $\mathrm{Cu}^{2+}$
B $\mathrm{Ca}$ and $\mathrm{Cr}^{3+}$
C $\mathrm{Ca}$ and $\mathrm{Ni}^{2+}$
D $\mathrm{Fe}^{3+}$ and $\mathrm{O}^{2-}$
▶️Answer/Explanation
Ans:A
Question
What number of protons, neutrons and electrons are present in the ion \(^{54}Fe^{3+}\)
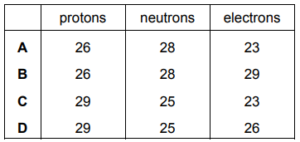
▶️Answer/Explanation
Ans:C
Question
Which ion has the same electronic configuration as $\mathrm{Cl}^{-}$?
A $\mathrm{F}^{-}$
B $\mathrm{P}^{+}$
C $\mathrm{Sc}^{3+}$
D $\mathrm{Si}^{4+}$
▶️Answer/Explanation
Ans:C
Question
Which ion has both more electrons than protons and more protons than neutrons? $\left[\mathrm{H}={ }_1^1 \mathrm{H} ; \mathrm{D}={ }_1^2 \mathrm{H} ; \mathrm{O}={ }_8^{16} \mathrm{O}\right]$
A $\mathrm{D}^{-}$
B $\mathrm{H}_3 \mathrm{O}^{+}$
C $\mathrm{OD}^{-}$
D $\mathrm{OH}^{-}$
▶️Answer/Explanation
Ans:D
Question
Which species contains the smallest number of electrons?
A $\mathrm{B}^{3+}$
B $\mathrm{Be}^{2+}$
C $\mathrm{H}^{-}$
D $\mathrm{He}^{+}$
▶️Answer/Explanation
Ans:D
Question
What is the electronic configuration of \(Mg^{2+}\)?
A \(1s^2 2s^2 2p^6\)
B \(1s^2 2s^2 2p^6 3s^2\)
C \(1s^2 2s^2 2p^6 3s^2 3p^2\)
D \(1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2\)
Answer/Explanation
Ans: A
Question
Which atom has its outermost electron in an orbital of the shape shown, with principal quantum number 3?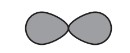
A sodium
B chlorine
C calcium
D bromine
Answer/Explanation
Ans: B
Question
Which atom has the same number of electrons as the hydroxide ion, \(OH^–\) ?
A F B Ne C Na D Mg
Answer/Explanation
Ans: B
Question
In which pair of species do both species have only one unpaired p electron?
A Ar+ and C– B B and Ti+ C F and Ga D Se–and Si–
Answer/Explanation
Answer: C
Question
The ion X2+ has the same electronic configuration as the atom Kr.
What is the electronic configuration of an atom of X?
A [Ar]4s²3d104p6
B [Ar]4s²3d104p65s² C [Ar]4s²4d104p6
D [Ar]4s²4d104p65s²
Answer/Explanation
Answer B
Question
Which statement about a 3p orbital is correct?
A It can hold a maximum of 6 electrons.
B It has the highest energy of the orbitals with principal quantum number 3.
C It is at a higher energy level than a 3s orbital but has the same shape.
D It is occupied by one electron in an isolated phosphorus atom.
Answer/Explanation
Answer:
D
Question
In which pairs do both species have the same number of unpaired electrons in p orbitals?
1 O and Cl+
2 F+ and Ga–
3 N and Kr3+
The responses A to D should be selected on the basis of

Answer/Explanation
Answer:
A
Question
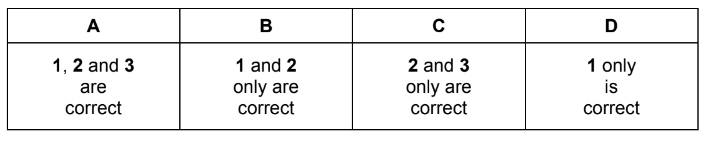
A particular atom of germanium, Ge, has a nucleon number of 70.
Which statements about \(_{32}^{70}\textrm{Ge}\) atom are correct?
1 It has the same number of electrons as a \(_{34}^{79}\textrm{Se}^{2+}\) ion.
2 It has the same number of neutrons as an atom of \(_{30}^{68}\textrm{Zn}\).
3 It has half as many protons as an atom of \(_{64}^{160}\textrm{Gd}\).
▶️Answer/Explanation
Answer A
Question
A particular atom of germanium, Ge, has a nucleon number of 70.
Which statements about \(_{32}^{70}\textrm{Ge}\) atom are correct?
1 It has the same number of electrons as a \(_{34}^{79}\textrm{Se}^{2+}\) ion.
2 It has the same number of neutrons as an atom of \(_{30}^{68}\textrm{Zn}\).
3 It has half as many protons as an atom of \(_{64}^{160}\textrm{Gd}\).

▶️Answer/Explanation
Answer A
Question
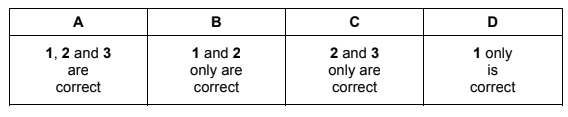
An atom of calcium-48 can form a 2+ ion.
Which statements about this ion are correct?
1 It has 20 protons.
2 It has 28 neutrons.
3 It has 22 electrons.
Answer/Explanation
Answer B
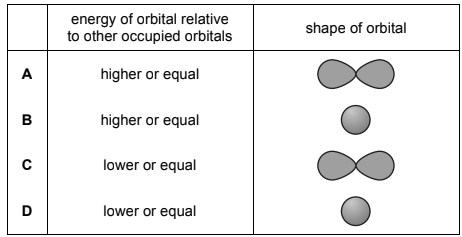
Question
The outermost electron in an atom of neon occupies a particular orbital. Which row shows the relative energy and shape of this orbital?
Answer/Explanation
Answer A
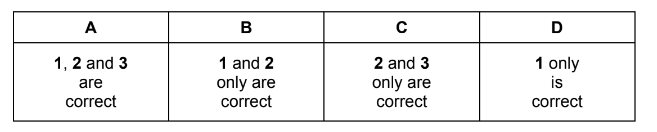
Question
Which ions contain one or more unpaired electrons?
1 Cu2+
2 Mn3+
3 V3+
Answer/Explanation
Answer A
Question
This question refers to isolated gaseous atoms.
In which atom are all electrons paired?
A Ba B Br C S D Si
Answer/Explanation
Answer A
Question
Drinking water may contain dissolved calcium hydrogencarbonate, Ca(HCO3)2.
How many electrons are present in a hydrogencarbonate anion?
A 30 B 31 C 32 D 33
Answer/Explanation
Answer:
C
Question
The electronic configuration of the two outermost shells of an atom is 3s23p63d54s2.
What is this atom?
A manganese
B phosphorus
C strontium
D vanadium
Answer/Explanation
Answer:
A
Question
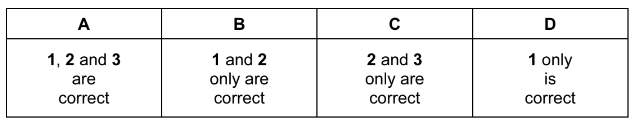
X is a particle with 18 electrons and 20 neutrons.
What could be the symbol of X?
1 \(_{18}^{38}\textrm{Ar}\)
2 \(_{20}^{40}\textrm{Ca}\)2+
3 \(_{19}^{39}\textrm{K}\)+
▶️Answer/Explanation
Answer: A
Question
Use of the Data Booklet is relevant to this question.
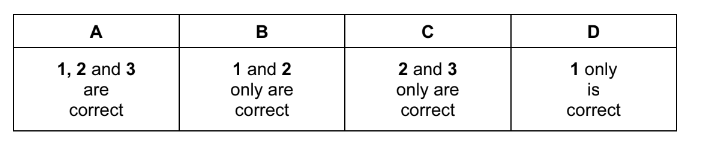
Which ions contain one or more unpaired electrons?
1\( Cu^{2+}\)
2\( Mn^[3+}\)
3 \(V^{3+}\)
The responses A to D should be selected on the basis of
▶️Answer/Explanation
Ans:A
Question
Use of the Data Booklet is relevant to this question.
Atoms of element X have six unpaired electrons.
What could be element X?
A carbon
B chromium
C iron
D selenium
Answer/Explanation
Ans:B
Question
Use of the Data Booklet is relevant to this question.
Free-radicals play an important part in reactions involving the destruction of the ozone layer and the substitution of alkanes by chlorine.
Some free-radicals contain two unpaired electrons. Such species are called diradicals. Which species are diradicals?
1 O
2 Cl
3 \(CH_3\)
The responses A to D should be selected on the basis of
No other combination of statements is used as a correct response.
Answer/Explanation
Ans: D
Question
Use of the Data Booklet is relevant to this question.
In which set do all species contain the same number of electrons?
A \(Co^{2+}, Co^{3+}, Co^{4+}\)
B \(F^–, Br^–, Cl^–\)
C \(Na^+, Mg^{2+}, Al^{3+}\)
D \(K_2SO_4, K_2SeO_4, K_2TeO_4\)
Answer/Explanation
Ans: C
Question
Use of the Data Booklet is relevant to this question.
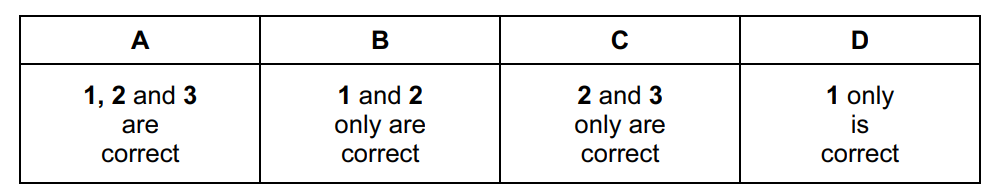
In which pairs do both species have the same number of unpaired p electrons?
- O and Cl+
- F+ and Ga–
- P and Ne+
Answer/Explanation
Ans:
B
