Question
Titanium is a transition element in Period 4. It is commonly found as TiO2 in minerals.
(a) (i) Define transition element.
(ii) Identify two typical properties of transition elements.
1 …………………………………………………………………………………………………………………………
2 …………………………………………………………………………………………………………………………
(b) The \(TiO^{2+}\) ion forms when \(TiO_2\) reacts with an excess of sulfuric acid.
\(TiO^{2+}\) can be reduced by zinc metal in acidic conditions to form a purple solution containing \(Ti^{3+}(aq)\).
(i) \(TiO^{2+}(aq)\) is a colourless ion.
Suggest why.
(ii) Give the electronic configuration of an isolated \(Ti^{3+}\) ion.
\(1s^2\)
(iii) Write an ionic equation for the reduction of \(TiO^{2+}\) by zinc metal in acidic conditions.
(c) Acidified \(Ti^{3+}(aq)\) reacts with oxygen dissolved in water as shown.
\(4Ti^{3+} + O_2 + 2H_2O → 4TiO^{2+} + 4H^+\) \( ∆G^o = –436.1kJmol^{–1}\)
The standard reduction potential, \(E^o\), of \(O_2 + 4H^+ + 4e^– \leftrightarrow 2H_2O\) is +1.23V.
(i) Calculate the standard reduction potential, \(E^o\), in V, of the \(TiO^{2+}(aq)/Ti^{3+}(aq)\) half-cell.
Show your working.
\(E^o = ………………………… V\)
(ii) When aqueous citrate ions, \(C_6H_5O_7^{3–}\), are added to \(Ti^{3+}(aq)\), the \([Ti(C_6H_5O_7)_2]^{3–}(aq)\) complex forms.
Explain, in terms of d-orbitals, why \(Ti^{3+}\) is able to form complex ions.
(iii) Acidified \([Ti(C_6H_5O_7)_2]^{3–}(aq)\) does not react with oxygen dissolved in water, unlike acidified \(Ti^{3+}(aq)\).
Suggest what this means for the value of the standard reduction potential, \(E^o\) , of the following half‐cell.
\([Ti(C_6H_5O_7)_2]^{2–}(aq) + e^– \leftrightarrow [Ti(C_6H_5O_7)_2]^{3–}(aq)\)
Explain your answer.
(d) Some reactions of \(TiO_2\) are shown in Fig. 3.1.
The anion, \(acac^–\), is a bidentate ligand.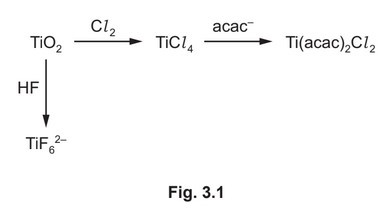
(i) The titanium ions in \(TiF_6^{2–}\) and \(Ti(acac)_2Cl_2\) have a coordination number of 6. State what is meant by coordination number.
(ii) Write an equation for the formation of \(TiF_6^{2–}\) from \(TiO_2\).
(iii) State what is meant by bidentate ligand.
(iv) \(Ti(acac)_2Cl_2\) shows both optical and geometrical (cis/trans) isomerism.
\(Ti(acac)_2Cl_2\) exists as three stereoisomers.
The structure of one stereoisomer of \(Ti(acac)_2Cl_2\) is shown in Fig. 3.2.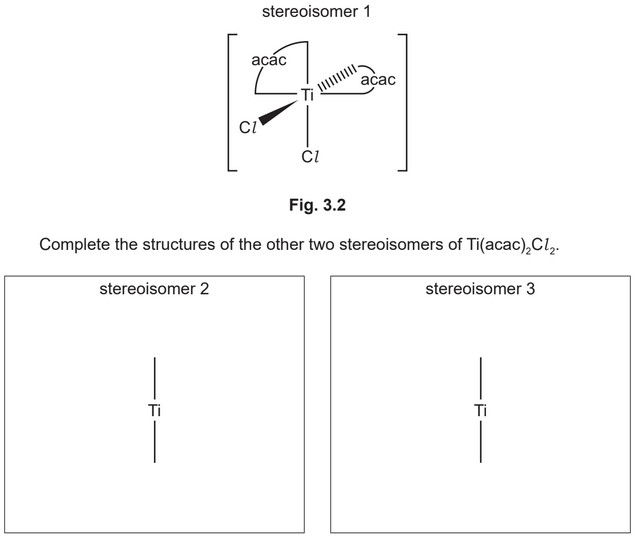
(v) The \(acac^–\) anion is symmetrical.
Deduce which, if any, of stereoisomers 1, 2 and 3 in (d)(iv) are polar.
Explain your answer.
Answer/Explanation
Answer:
(a) (i) (d-block) element that forms one or more stable ions with incomplete d subshell / incomplete d orbitals
(ii) • variable oxidation states
• behave as catalysts
• form complex ions / complexes
• form coloured compounds / ions
Any two for one mark
(b) (i) Ti is in +4 oxidation state so no d electrons / \(d^0\) OR Ti in \(TiO^{2+}\) has no d electrons / d0 [1]
cannot absorb photons / light in visible spectrum OR no wavelength / frequency absorbed in visible spectrum [1]
(ii) \((1s^2) 2s^2 2p^6 3s^2 3p^6 3d^1 (4s^0)\)
(iii) \(2TiO^{2+} + 4
H^+ + Zn
→ 2Ti^{3+} + 2H_2O + Zn^{2+}\)
(c) (i) M1: ∆G = \(– nE⦵_{cell}F\) AND n = 4
M2: ∴ \(E⦵_{cell}\) = –436100 / –4(96500) = 1.13 V ecf
M3: \(E⦵_{cell} = E^⦵(O_2,4H^+|H^2O) – E^⦵(TiO^{2+}|Ti^{3+}) = 1.23 – E^⦵(TiO^{2+}|Ti^{3+})\)
∴ \(E⦵(TiO^{2+}|Ti^{3+}) = (+)0.1 (V) ecf\)
(ii) \(Ti^{3+}\) empty / vacant d orbitals can form dative bonds / accept a lone pair from a ligand
OR \(Ti^{3+}\) has vacant d-orbitals which are energetically accessible
(iii) the E⦵ of the half-cell must be greater than +1.23 V / E⦵ of the \(O_2|H^+\) half-cell as E⦵cell< 0 and the reaction does not occur
(d) (i) the number of co-ordinate bonds being formed by the metal atom/ion
(ii) \(TiO_2 + 6HF → TiF_6^{2–} + 2H_2O + 2H^+\)
OR \(TiO_2 + 6HF → TiF_6^{2–} + 2H_3O^+\)
(iii) species with two lone pairs (of electrons) [1]
that form dative covalent / co-ordinate bonds to a central metal atom/ion [1]
(iv) 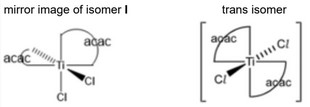
(v) isomer I AND cis isomer drawn by candidate ecf [1]
dipoles do not cancel / partial charges do not cancel [1]
