Question:
Which row is correct?
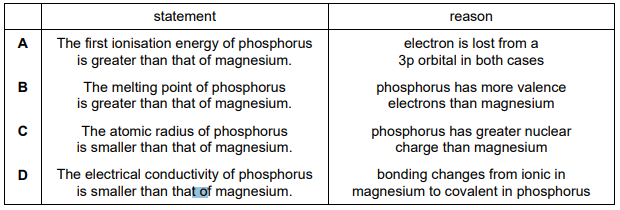
▶️Answer/Explanation
Ans:C
Question
The definitions of many chemical terms can be illustrated by chemical equations.
Which terms can be illustrated by an equation that includes the formation of a positive ion?
1 first ionisation energy
2 heterolytic fission of a covalent bond
3 enthalpy change of atomisation
▶️Answer/Explanation
Ans:B
Question
For the element sulfur, which pair of ionisation energies has the largest difference between them?
A third and fourth ionisation energies
B fourth and fifth ionisation energies
C fifth and sixth ionisation energies
D sixth and seventh ionisation energies
▶️Answer/Explanation
Ans:D
Question
The first ionisation energy of potassium, K, is 418 kJ \(mol^{–1}\). The first ionisation energy of strontium, Sr, is 548 \(kJmol^{–1}\).
Which statement helps to explain why Sr has a greater first ionisation energy than K?
A The charge on a Sr nucleus is greater than the charge on a K nucleus.
B The outer electron in a Sr atom experiences greater shielding than the outer electron in a K atom.
C The outer electron in a Sr atom experiences spin-pair repulsion.
D The outer electron in a Sr atom is further from the nucleus than the outer electron in a K atom.
Answer/Explanation
Ans: A
Question
Why is the first ionisation energy of oxygen less than that of nitrogen?
A The nitrogen atom has its outer electron in a different subshell.
B The nuclear charge on the oxygen atom is greater than that on the nitrogen atom.
C The oxygen atom has a pair of electrons in one p orbital that repel one another.
D There is more shielding in an oxygen atom.
Answer/Explanation
Answer C
Question
Which of these elements has the highest fifth ionisation energy?
A C B N C P D Si
Answer/Explanation
Answer A
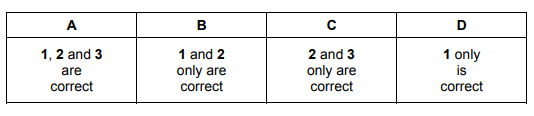
Question
The first ionisation energy of chlorine is greater than that of sulfur.
Which factors contribute to this?
1 Chlorine has more protons in its nucleus than sulfur does.
2 Chlorine has greater electron shielding than sulfur does.
3 The covalent bonds in chlorine molecules are stronger than those in sulfur molecules.
▶️Answer/Explanation
Answer D
Question
Element X has a higher first ionisation energy than element Y.
Two students state what they believe is one factor that helps to explain this.
student 1 “X has a higher first ionisation energy than Y because an atom of X has more protons in its nucleus than an atom of Y.”
student 2 “X has a higher first ionisation energy than Y because X has a smaller atomic radius than Y.”
Only one of the two students is correct.
What could X and Y be?
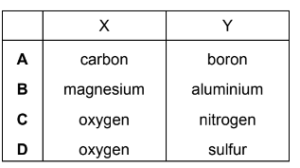
Answer/Explanation
Answer D
Question
The definitions of many chemical terms can be illustrated by chemical equations.
Which terms can be illustrated by an equation that includes the formation of a positive ion?
1 first ionisation energy
2 heterolytic fission of a covalent bond
3 enthalpy change of atomisation
The responses A to D should be selected on the basis of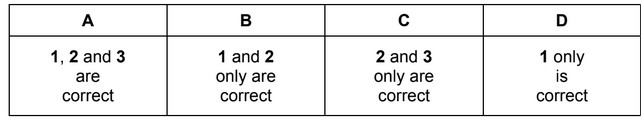
Answer/Explanation
Ans: B
Question
Why is the second ionisation energy of sodium larger than the second ionisation energy of magnesium?
A The attraction between the nucleus and the outer electron is greater in \(Na^+\) than in \(Mg^+\).
B The nuclear charge of \(Na^+\) is greater than that of \(Mg^+\).
C The outer electron of \(Na^+\) is more shielded than the outer electron of \(Mg^+\).
D The outer electron of Na is in the same orbital as the outer electron of Mg.
Answer/Explanation
Ans: A
Question
The first four ionisation energies for element X are shown in the table.
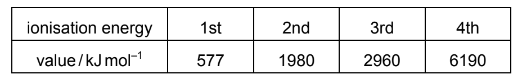
Which ion of X is produced by removing an electron from a filled shell?
A X+ B X2+ C X3+ D X4+
Answer/Explanation
Answer D
Question
The relative first ionisation energies of four elements with consecutive atomic numbers below 20 are shown on the graph. One of the elements reacts with hydrogen to form a covalent compound with formula HX.
Which element could be X?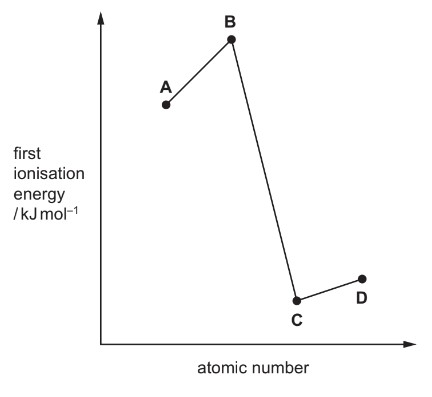
Answer/Explanation
Ans: A
Question
Which property of an atom does not affect its first ionisation energy?
A the atomic radius
B the number of electron shells
C the number of neutrons
D the number of protons
Answer/Explanation
Ans: C
Question
The table gives the successive ionisation energies for an element X.
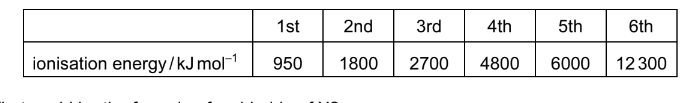
What could be the formula of a chloride of X?
A XCl B \( XCl _{2}\) C \(XCl _{3}\) D \( XCl_{ 4}\)
Answer/Explanation
Ans:C
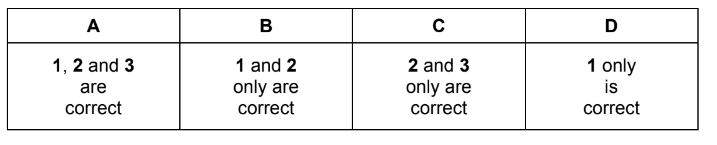
Question
The first ionisation energies of twenty successive elements in the Periodic Table are represented in the graph.
The letters given are not the normal symbols for these elements.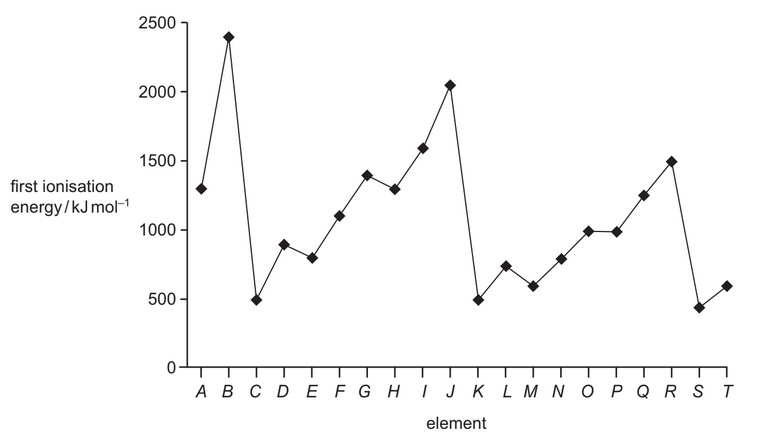
Which statements about this graph are correct?
1 Elements B, J and R are in Group 0 of the Periodic Table.
2 Atoms of elements D and L contain two electrons in their outer shells.
3 Atoms of elements G and O contain a half-filled p subshell.
The responses A to D should be selected on the basis of
No other combination of statements is used as a correct response.
Answer/Explanation
Ans: A
Question
Use of the Data Booklet is relevant to this question.
The elements radon (Rn), francium (Fr) and radium (Ra) have proton numbers 86, 87 and 88 respectively.
What is the order of their first ionisation energies?
Answer/Explanation
Ans: A
Question
What are the trends in the stated properties as Group II is descended from magnesium to barium?
Answer/Explanation
Ans:
C
Question
The value of the second ionisation energy of calcium is 1150kJ mol–1.
Which equation correctly represents this statement?
- Ca(g) → Ca2+(g) + 2e− ; ∆H
o= +1150kJmol–1 - Ca+(g) → Ca2+(g) + e− ; ∆H
o= +1150kJmol–1 - Ca+(g) → Ca2+(g) + e− ; ∆H
o= –1150kJmol–1 - Ca(g) → Ca2+(g) + 2e− ; ∆H
o= –1150kJmol–1
Answer/Explanation
Ans:
B
