The Group 14 elements show a change from non-metallic to metallic character down the group.
(a) Table 3.1 shows some properties of two Group 14 elements, C and Sn, in their standard states. The table is incomplete.
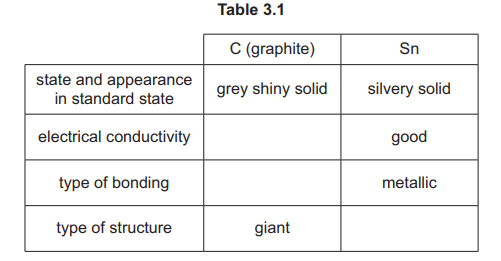
(i) Complete Table 3.1.
(ii) Identify the lattice structure shown by graphite.
(iii) Explain why Sn has good electrical conductivity.
(b) Carbon is found in inorganic compounds such as carbonates.
(i) Write an equation for the reaction of magnesium carbonate with dilute HCl(aq).
(ii) Describe the thermal stability of the carbonates down Group 2.
(iii) Ammonium carbonate undergoes an acid–base reaction with NaOH(aq). Explain this statement.
(c) Fig. 3.1 shows a sketch of some of the ionisation energies of silicon, Si.
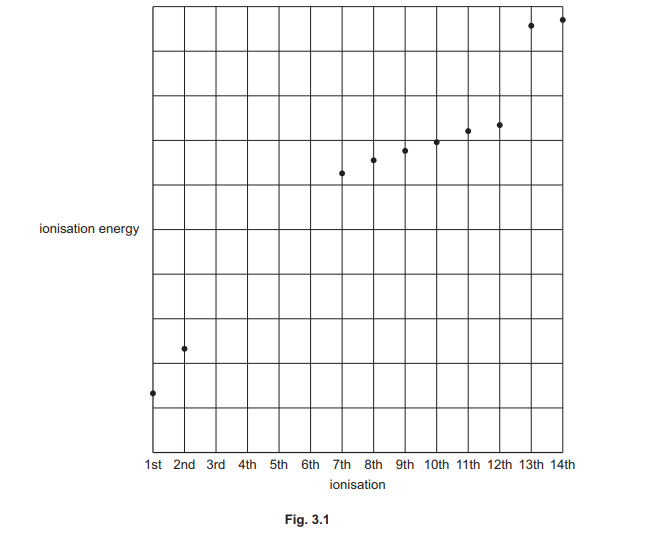
(i) Complete the graph in Fig. 3.1 to show the third to sixth ionisation energies of Si.
(ii) Construct an equation to represent the second ionisation energy of Si.
(d) Fig. 3.2 shows the boiling points of the simplest hydrides of the Group 14 elements, C to Pb.
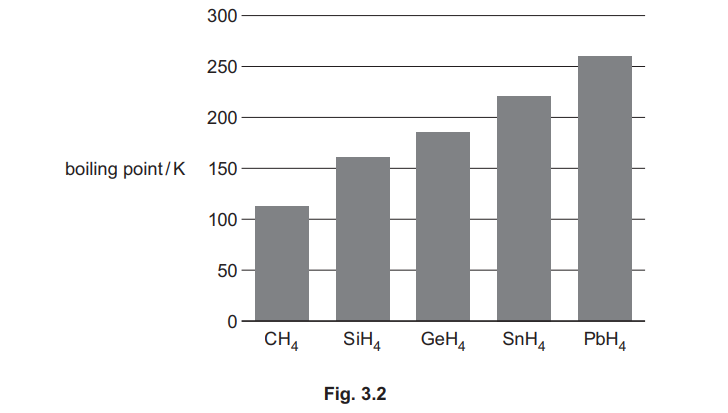
(i) Explain the trend in the boiling points of the Group 14 hydrides shown in Fig. 3.2.
(ii) Deduce the shape of a molecule of SiH₄.
(e) Silicon readily reacts with elements of high electronegativity.
(i) Write an equation for the formation of SiCl₄ from its constituent elements.
(ii) Describe what is observed when a small sample of SiCl₄ is added to water.
(iii) SiO₂ is a white solid that melts above 1700°C. SiCl₄ is a colourless liquid at room temperature. Explain the difference in the melting points of these two compounds with reference to their structure and bonding.
(f) Tin forms an amphoteric oxide, SnO₂. Suggest the formula of the tin compound that forms when SnO₂ reacts with H₂SO₄ in an acid–base reaction.
▶️ Answer/Explanation
(a)(i) Explanation: Carbon (C) is a non-metal with a giant covalent structure (graphite), while tin (Sn) is a metal with metallic bonding. Graphite conducts electricity due to delocalized electrons, whereas Sn conducts due to free electrons in its metallic lattice.
(a)(ii) Layer/lattice structure.
(a)(iii) Explanation: Tin has good electrical conductivity because it is a metal with delocalized electrons that can move freely and carry charge.
(b)(i) MgCO₃ + 2HCl → MgCl₂ + H₂O + CO₂
(b)(ii) Explanation: Thermal stability of Group 2 carbonates increases down the group due to decreasing polarizing power of the cation, which reduces decomposition into oxide and CO₂.
(b)(iii) Explanation: Ammonium carbonate acts as an acid, donating protons (H⁺) to NaOH, forming water and ammonia (NH₃).
(c)(i) Graph: The ionisation energies increase progressively, with a sharp rise after the 4th ionisation (due to removal of core electrons).
(c)(ii) Si⁺(g) → Si²⁺(g) + e⁻
(d)(i) Explanation: Boiling points increase from CH₄ to PbH₄ due to stronger London dispersion forces as molecular size and electron count increase.
(d)(ii) Tetrahedral.
(e)(i) Si + 2Cl₂ → SiCl₄
(e)(ii) Effervescence/misty fumes (due to HCl formation).
(e)(iii) Explanation: SiO₂ has a giant covalent structure requiring high energy to break bonds, whereas SiCl₄ has weak intermolecular forces, making it a low-melting liquid.
(f) Sn(SO₄)₂
