Chlorine is one of the elements in Group 17 of the Periodic Table.
(a) (i) Describe the colours of the Group 17 elements, chlorine to iodine, at room temperature.
(ii) Describe the relative reactivity of the elements chlorine to iodine as oxidising agents.
(iii) State what is observed when chlorine reacts with hydrogen.
(iv) Explain why the thermal stability of the hydrogen halides decreases down the group.
(b) The halogenoalkane CH₃CH₂Cl forms when chlorine reacts with C₂H₆ via a free-radical substitution mechanism.
(i) Define free radical.
(ii) State the essential condition for chlorine to react with \(C_2H_6\) at room temperature.
(iii) Write two equations to show the propagation steps in this reaction.
(c) CHCl₃ is another halogenoalkane. CHCl₃ forms when propanone reacts with NaClO. NaClO is made from chlorine in a disproportionation reaction.
(i) Identify a reagent and conditions that can be used to convert chlorine to NaClO.
(ii) Define disproportionation.
(iii) Write numbers in the boxes to balance the equation showing the reaction of propanone with NaClO.
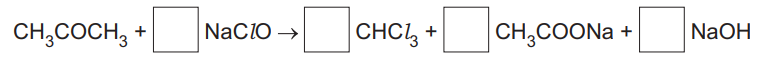
(iv) Aqueous AgNO₃ dissolved in ethanol reacts with an aqueous solution of CHCl₃. State what is observed in this reaction. Explain your answer.
▶️ Answer/Explanation
(a)(i) Chlorine is yellow-green, bromine is orange/brown/red, and iodine is silver-grey/black at room temperature.
Explanation: The colour of halogens darkens down the group due to increasing molecular size and electronic transitions.
(a)(ii) The oxidising strength decreases from chlorine to iodine.
Explanation: Chlorine has the highest electron affinity and strongest oxidising ability, which decreases down the group as atomic size increases.
(a)(iii) The green colour of chlorine disappears.
Explanation: Chlorine reacts with hydrogen to form colorless hydrogen chloride (HCl), removing the green colour.
(a)(iv) The H—Hal bond strength decreases down the group.
Explanation: As the halogen size increases, bond length increases, weakening the bond and reducing thermal stability.
(b)(i) A free radical is a species with one or more unpaired electrons.
Explanation: Free radicals are highly reactive due to the presence of unpaired electrons.
(b)(ii) Ultraviolet (UV) light is required.
Explanation: UV light provides the energy to break the Cl—Cl bond, initiating the free-radical reaction.
(b)(iii) Propagation steps:
1. \( \text{Cl}^\cdot + \text{C}_2\text{H}_6 \rightarrow \text{HCl} + \text{C}_2\text{H}_5^\cdot \)
2. \( \text{C}_2\text{H}_5^\cdot + \text{Cl}_2 \rightarrow \text{C}_2\text{H}_5\text{Cl} + \text{Cl}^\cdot \)
Explanation: In propagation, radicals react to form products while regenerating new radicals.
(c)(i) Reagent: Cold dilute NaOH. Conditions: Room temperature.
Explanation: Chlorine disproportionates in cold dilute NaOH to form NaClO (sodium hypochlorite).
(c)(ii) Disproportionation is a reaction where the same element is both oxidised and reduced.
Explanation: In \( \text{Cl}_2 + 2\text{NaOH} \rightarrow \text{NaCl} + \text{NaClO} + \text{H}_2\text{O} \), chlorine is both oxidised (+1 in NaClO) and reduced (-1 in NaCl).
(c)(iii) Balanced equation:
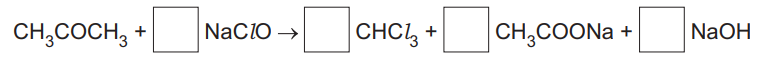
Explanation: The balanced equation is \( \text{CH}_3\text{COCH}_3 + 3\text{NaClO} \rightarrow \text{CHCl}_3 + \text{CH}_3\text{COONa} + 2\text{NaOH} \).
(c)(iv) No visible reaction (no precipitate forms).
Explanation: CHCl₃ does not contain a replaceable halogen (C—Cl bonds are strong), so no AgCl precipitate forms with AgNO₃.
