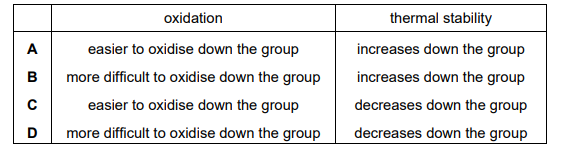
The table refers to the hydrogen halides. Which row is correct?
▶️ Answer/Explanation
Ans: C
Hydrogen halides (HX) exhibit trends in thermal stability and bond strength:
- Thermal stability decreases down the group (HF > HCl > HBr > HI).
- Bond strength decreases down the group (HF has the strongest bond, HI the weakest).
- Reducing power increases down the group (HI is the strongest reducing agent).
Row C correctly states that hydrogen iodide (HI) is the strongest reducing agent and decomposes at the lowest temperature, making it the correct answer.
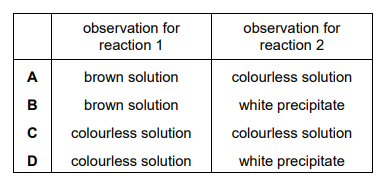
In reaction 1, concentrated sulfuric acid is added to potassium chloride and the fumes produced are bubbled into aqueous potassium iodide solution. In reaction 2, potassium chloride is dissolved in aqueous ammonia and this is then added to aqueous silver nitrate. What are the observations for reactions 1 and 2?
▶️ Answer/Explanation
Ans: C
Reaction 1: Concentrated \( \text{H}_2\text{SO}_4 \) oxidizes \( \text{Cl}^- \) to \( \text{HCl(g)} \), which reacts with \( \text{KI} \) to produce \( \text{I}_2 \) (purple fumes). Reaction 2: \( \text{AgNO}_3 \) reacts with \( \text{Cl}^- \) in \( \text{NH}_3 \) to form \( \text{AgCl} \) (white precipitate). Thus, the correct observations are purple fumes (1) and white precipitate (2), matching option C.
Which statement comparing magnesium and barium, or their compounds, is correct?
▶️ Answer/Explanation
Ans: C
Let’s analyze each option:
A: Incorrect. Reactivity with acids increases down Group 2. Barium reacts more vigorously with HCl than magnesium.
B: Incorrect. Both \(MgCO_3\) and \(BaCO_3\) produce 1 mole of \(CO_2\) per mole of carbonate when reacting with excess HCl.
C: Correct. \(MgSO_4\) is highly soluble, while \(BaSO_4\) is practically insoluble in water.
D: Incorrect. Thermal stability of carbonates decreases down Group 2. \(MgCO_3\) decomposes more readily than \(BaCO_3\).
Thus, C is the only correct statement.
X is a Group 2 element in either Period 3 or Period 5. X(OH)₂ is less soluble in water than Ca(OH)₂. When X(NO₃)₂ is heated, it decomposes. Which row is correct?

▶️ Answer/Explanation
Ans: B
To determine the correct row:
- Solubility trend: In Group 2, solubility of hydroxides increases down the group. Since \(X(OH)_2\) is less soluble than \(Ca(OH)_2\), \(X\) must be above calcium in the group (i.e., magnesium, Period 3).
- Thermal decomposition: Group 2 nitrates decompose on heating to form the metal oxide, nitrogen dioxide, and oxygen: \[ 2X(NO_3)_2(s) \to 2XO(s) + 4NO_2(g) + O_2(g) \] This applies to all Group 2 nitrates, confirming \(X\) is a Group 2 element.
Thus, the correct row is B (Period 3, decomposes).
