Nitrogen and phosphorus are elements in Group 15 of the Periodic Table.
(a) Nitrogen is found in inorganic compounds such as nitrogen oxides (\(NO_x\)), nitrates and nitric acid.
(i) Identify one natural and one man-made occurrence of nitrogen oxides in the atmosphere.
(ii) Write an equation to describe the role of NO₂ in the direct formation of acid rain.
(iii) Peroxyacetyl nitrate, PAN, is a component of photochemical smog. Describe how PAN forms from NO₂.
(iv) Nitric acid reacts with basic oxides to form nitrates. Write an equation for the reaction of nitric acid with calcium oxide.
(v) Describe what is seen when solid calcium nitrate is heated strongly.
(b) A common test for nitrates is the reaction with NaOH and Al. Equation 1 shows the reaction.
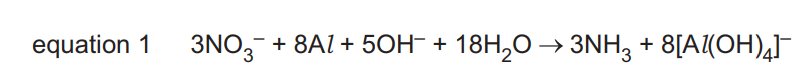
(i) Deduce the oxidation state of nitrogen in \(NO_3^–\).
(ii) Identify the species that is oxidised in equation 1.
(iii) NH3 is a basic gas. Describe how NH₃ is able to act as a base.
(iv) Suggest the shape of the [Al(OH)₄]⁻ ion.
(c) Fig. 3.1 shows a sketch of some of the ionisation energies of phosphorus, P.
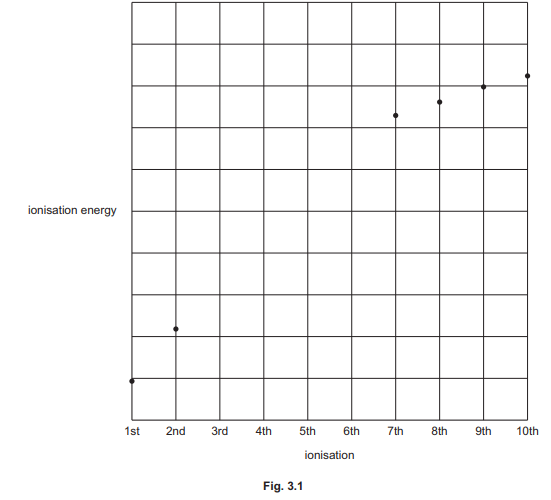 (i) Construct an equation to represent the third ionisation energy of P.
(i) Construct an equation to represent the third ionisation energy of P.
(ii) Complete the graph in Fig. 3.1 to show the third to sixth ionisation energies of P.
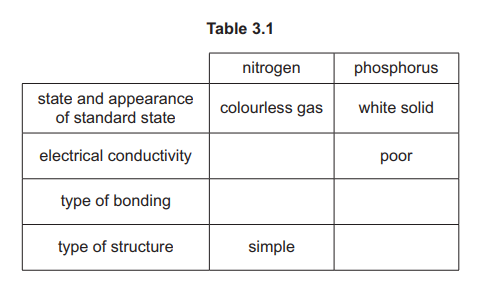
(d) Complete Table 3.1 to show the properties of nitrogen and phosphorus in their standard states.
(e) A form of solid nitrogen has a lattice structure similar to solid iodine. Identify the type of lattice structure of solid nitrogen.
(f) At very high temperatures, phosphorus can form \(P_2\) molecules. \(P_2\) contains a triple bond, \(P \equiv P\).
(i) Describe the formation of the \(P \equiv P\) bond in terms of orbital overlap.
(ii) The bond energy of \(P \equiv P\) is 485 kJ mol⁻¹. The bond energy of \(N \equiv N\) is 944 kJ mol⁻¹. Compare the reactivity of \(P_2\) and \(N_2\). Explain your answer.
▶️ Answer/Explanation
(a)(i) Natural: Lightning discharges. Man-made: Combustion of fossil fuels.
Explanation: Nitrogen oxides form naturally during lightning strikes due to high temperatures, while man-made sources include vehicle emissions and industrial processes.
(a)(ii) \(2NO_2 + H_2O \rightarrow HNO_3 + HNO_2\)
Explanation: NO₂ reacts with water to form nitric acid (HNO₃) and nitrous acid (HNO₂), contributing to acid rain.
(a)(iii) PAN forms when NO₂ reacts with volatile organic compounds (VOCs) in sunlight.
Explanation: Photochemical smog involves NO₂ and hydrocarbons undergoing reactions under sunlight to produce PAN.
(a)(iv) \(2HNO_3 + CaO \rightarrow Ca(NO_3)_2 + H_2O\)
Explanation: Nitric acid neutralizes calcium oxide, forming calcium nitrate and water.
(a)(v) Brown fumes of NO₂ gas are observed.
Explanation: Heating calcium nitrate decomposes it into calcium oxide, nitrogen dioxide (NO₂), and oxygen.
(b)(i) +5
Explanation: The oxidation state of N in \(NO_3^-\) is calculated as \(x + 3(-2) = -1\), giving \(x = +5\).
(b)(ii) Aluminium (Al)
Explanation: Al is oxidized from 0 to +3, losing electrons in the reaction.
(b)(iii) NH₃ donates a lone pair of electrons to protons (H⁺).
Explanation: Ammonia acts as a base by accepting protons using its lone pair on nitrogen.
(b)(iv) Tetrahedral
Explanation: The [Al(OH)₄]⁻ ion has four bonding pairs around Al, adopting a tetrahedral geometry.
(c)(i) \(P^{2+}(g) \rightarrow P^{3+}(g) + e^-\)
Explanation: The third ionisation energy removes an electron from \(P^{2+}\) to form \(P^{3+}\).
(c)(ii) Graph shows increasing ionisation energies with a large jump after the 5th ionisation.
Explanation: The graph reflects the removal of electrons from inner shells, requiring more energy.
(d) See image for completed table.
Explanation: Nitrogen is a diatomic gas (N₂), while phosphorus exists as P₄ molecules in a solid state.
(e) Molecular lattice
Explanation: Solid nitrogen, like iodine, forms a molecular lattice held by weak van der Waals forces.
(f)(i) Overlap of three p-orbitals from each P atom forms a triple bond.
Explanation: The \(P \equiv P\) bond involves one sigma and two pi bonds from p-orbital overlap.
(f)(ii) \(P_2\) is more reactive than \(N_2\) due to its weaker triple bond (485 vs. 944 kJ mol⁻¹).
Explanation: The lower bond energy of \(P \equiv P\) makes it easier to break, increasing reactivity compared to \(N \equiv N\).