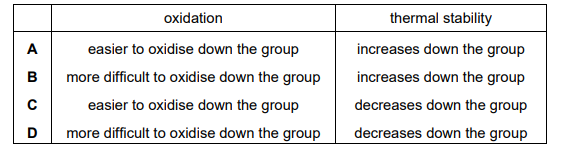
The table refers to the hydrogen halides. Which row is correct?
▶️ Answer/Explanation
Ans: C
Hydrogen halides (HX) exhibit trends in thermal stability and acidity:
- Thermal stability decreases down the group (HF > HCl > HBr > HI) due to weaker H-X bonds.
- Acidity increases down the group (HF < HCl < HBr < HI) because bond strength decreases, making proton release easier.
Row C correctly states that HI is the least thermally stable and most acidic, matching the observed trends.
A sample consisting of 1.0 mol of anhydrous calcium nitrate is completely decomposed by strong heating. What is the total amount of gas produced in this reaction?
▶️ Answer/Explanation
Ans: C
The decomposition reaction of calcium nitrate (\( \text{Ca(NO}_3\text{)_2} \)) upon strong heating is:
\[ 2\text{Ca(NO}_3\text{)_2} \rightarrow 2\text{CaO} + 4\text{NO}_2 + \text{O}_2 \]
For 1.0 mol of \( \text{Ca(NO}_3\text{)_2} \), the gas products are:
- \( 2.0 \, \text{mol} \) of \( \text{NO}_2 \)
- \( 0.5 \, \text{mol} \) of \( \text{O}_2 \)
Total gas produced = \( 2.0 + 0.5 = 2.5 \, \text{mol} \). Hence, the correct answer is C.
Compound Q dissolves in water. Q(aq) does not react with dilute sulfuric acid. Q(aq) forms a precipitate when aqueous silver nitrate is added. This precipitate is partially soluble in aqueous ammonia. What could be compound Q?
▶️ Answer/Explanation
Ans: C
1. No reaction with dilute \(H_2SO_4\) rules out barium compounds (A and B), as barium forms an insoluble sulfate precipitate.
2. Precipitate with \(AgNO_3\) suggests a halide (Br⁻ or I⁻).
3. Partial solubility in \(NH_3(aq)\) indicates the halide is bromide (AgBr is partially soluble, while AgI is insoluble).
4. Magnesium bromide (C) fits all criteria, as it dissolves in water, does not react with \(H_2SO_4\), and forms AgBr, which is partially soluble in ammonia.
A solid sodium halide, NaX, is reacted with concentrated sulfuric acid. The lowest oxidation state of sulfu)r in the products is +4. Halogen Y₂ is less volatile than halogen X₂. What are the identities of sodium halide NaX and halogen Y₂?

▶️ Answer/Explanation
Ans: B
When a sodium halide (NaX) reacts with concentrated sulfuric acid (H₂SO₄), sulfur is reduced to its lowest oxidation state of +4 (in SO₂). This occurs with NaBr (sodium bromide), where bromide ions (Br⁻) are oxidized to bromine (Br₂). Since Y₂ is less volatile than X₂, and iodine (I₂) is less volatile than bromine (Br₂), the correct pair is NaBr and I₂ (option B).
