Phosphoric(V) acid, H₃PO₄, is used in both inorganic and organic reactions.
(a) H₃PO₄ is made in a two-step process from phosphorus.
step 1 Phosphorus reacts with an excess of oxygen to form a white solid.
step 2 The white solid then reacts with water to form H₃PO₄.
(i) Write an equation for each step.
(ii) H₃PO₄ is a weak Brønsted–Lowry acid. Define weak Brønsted–Lowry acid.
(b) H₃PO₄ is also formed in the process shown in reaction 1.
reaction 1 \(4H_3PO_3 \to 3H_3PO_4 + PH_3\)
Table 3.1 shows some relevant thermodynamic data.
(i) Define enthalpy change of formation.
(ii) Use the data in Table 3.1 to calculate the enthalpy change, \(\Delta H_r\), of reaction 1.
(iii) Explain why reaction 1 is a disproportionation reaction. Explain your reasoning with reference to relevant oxidation numbers.
(c) Fig. 3.1 shows a reaction scheme that involves H₃PO₄ in several reactions.
(i) Identify A, which reacts with propene in the presence of H₃PO₄ in reaction 2.
(ii) Draw the structure of B.
(iii) Name the type of reaction that occurs in reaction 3.
(iv) Reaction 3 is monitored using infrared spectroscopy. It is not possible to use the O—H absorption frequency to monitor the reaction. Use Table 3.2 to identify a suitable bond whose absorption frequency can be used to monitor the progress of reaction 3. State the change you would see in the infrared spectrum during reaction 3.
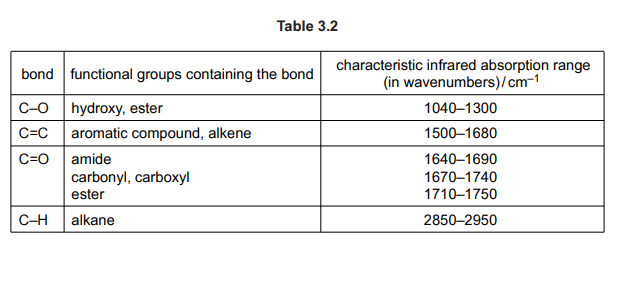
(d) H₃PO₄ also reacts with alcohols to form organophosphates. Organophosphates are compounds similar to esters. They have the general structure shown in Fig. 3.2.
(i) Complete the equation to suggest the products of the reaction of H₃PO₄ with methanol, CH₃OH.
(ii) Compound T is a simple organophosphate. The mass spectrum of T shows a molecular ion peak at m/e = 182. This peak has a relative intensity of 12.7. The relative intensity of the M+1 peak is 0.84. Deduce the number of carbon atoms in T. Hence suggest the molecular formula of T. Assume that phosphorus and oxygen exist as single isotopes. Show your working.
▶️ Answer/Explanation
(a)(i)
Step 1: \(4P + 5O_2 \to P_4O_{10}\)
Step 2: \(P_4O_{10} + 6H_2O \to 4H_3PO_4\)
Explanation: Phosphorus reacts with oxygen to form phosphorus pentoxide (\(P_4O_{10}\)), which then hydrolyzes with water to produce phosphoric acid (\(H_3PO_4\)).
(a)(ii) A weak Brønsted–Lowry acid is a proton (H⁺) donor that partially dissociates in solution.
Explanation: \(H_3PO_4\) does not fully ionize in water, releasing only a fraction of its protons.
(b)(i) Enthalpy change of formation is the energy change when one mole of a compound is formed from its constituent elements in their standard states.
(b)(ii) \(\Delta H_r = +9 + 3(-1281) – 4(-972) = +54 \text{ kJ mol}^{-1}\)
Explanation: The enthalpy change is calculated using the given stoichiometry and standard enthalpies of formation.
(b)(iii) This is a disproportionation reaction because phosphorus in \(H_3PO_3\) is both oxidized (to \(H_3PO_4\)) and reduced (to \(PH_3\)).
Explanation: The oxidation state of P changes from +3 to +5 (oxidation) and +3 to -3 (reduction).
(c)(i) A is \(H_2O\) (water/steam).
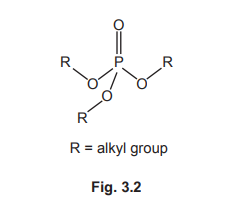
(c)(ii) Structure of B:
(c)(iii) The reaction type is condensation.
(c)(iv) Bond: C=O
Change in infrared spectrum: The absorption shifts from 1670–1740 cm⁻¹ to 1710–1750 cm⁻¹.
(d)(i) \(H_3PO_4 + 3CH_3OH \to (CH_3O)_3PO + 3H_2O\)
(d)(ii) Number of carbon atoms = \(\frac{0.84}{12.7} \times \frac{100}{1.1} = 6\)
Molecular formula: \(C_6H_{15}O_4P\)
Explanation: The M+1 peak intensity ratio is used to determine the number of carbon atoms, leading to the molecular formula.
