Oxygen is a Group 16 element.
(a) (i) Write equations for the following reactions.
• sodium and oxygen
• sulfur and oxygen
(ii) Draw a dot-and-cross diagram to show the species present in Al₂O₃. Draw outer electrons only.
(iii) The maximum oxidation state of the Period 3 elements in their oxides varies across the period. State and explain the variation.
(b) \(H_2O\) reacts with both inorganic and organic compounds.
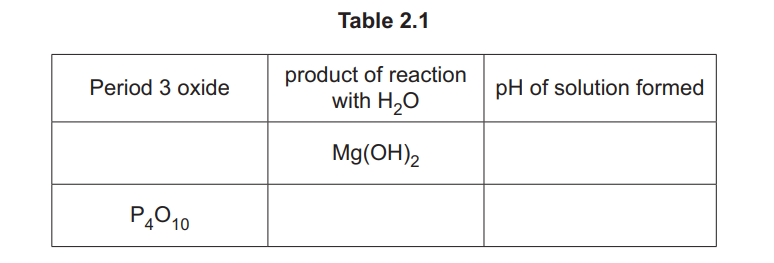
(i) Complete Table 2.1 to give details of the reactions of some Period 3 oxides with \(H_2O\).
(ii) Write an equation for the reaction of CH₃CN with H₂O in acidic conditions. 
(iii) Draw the structures of the two alcohols formed in the reaction shown in equation 1. 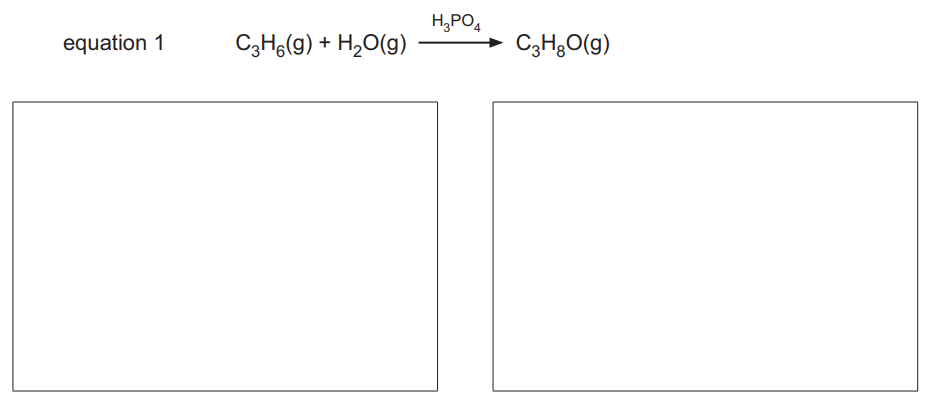
(iv) Explain why alcohols are less acidic than water.
(c) Fig. 2.1 shows the boiling points of \(H_2O\) and other Group 16 hydrides.
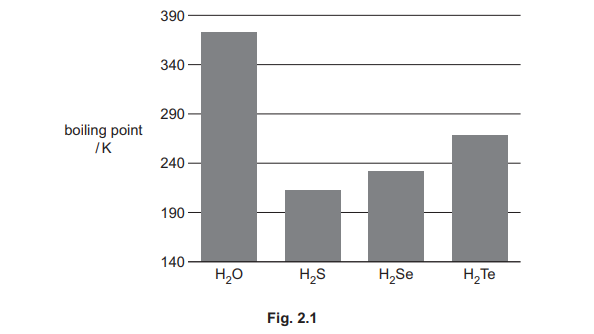
(i) Explain the trend in the boiling points of the Group 16 hydrides H₂S to H₂Te.
(ii) Explain why the boiling point of \(H_2O\) is much higher than that of \(H_2S\).
▶️ Answer/Explanation
(a)(i)
• Sodium and oxygen: \(4Na + O_2 \rightarrow 2Na_2O\)
• Sulfur and oxygen: \(S + O_2 \rightarrow SO_2\)
Explanation: Sodium reacts with oxygen to form sodium oxide (\(Na_2O\)), while sulfur burns in oxygen to produce sulfur dioxide (\(SO_2\)).
(a)(ii)
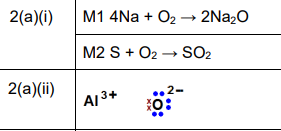
Explanation: The dot-and-cross diagram for \(Al_2O_3\) shows aluminum donating 3 electrons and oxygen accepting 2 electrons, forming an ionic lattice.
(a)(iii)
The maximum oxidation state increases across Period 3.
Explanation: As the number of valence electrons increases, elements can lose, share, or donate more electrons, leading to higher oxidation states in their oxides.
(b)(i)
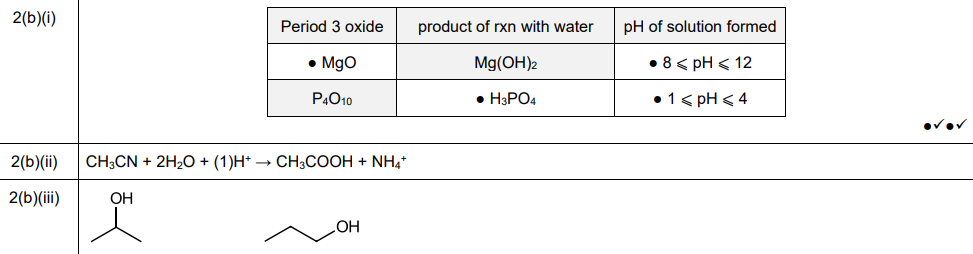
Explanation: The table is completed with the correct reactions of Period 3 oxides with water, including their pH and product types.
(b)(ii)
\(CH_3CN + 2H_2O \rightarrow CH_3COOH + NH_3\) (in acidic conditions)
Explanation: Acetonitrile (\(CH_3CN\)) hydrolyzes in acidic water to form acetic acid (\(CH_3COOH\)) and ammonia (\(NH_3\)).
(b)(iii)
Structures: Methanol (\(CH_3OH\)) and Ethanol (\(C_2H_5OH\)).
Explanation: The reaction produces two alcohols, methanol and ethanol, as shown in the given equation.
(b)(iv)
Alcohols are less acidic than water because alkyl groups are electron-donating, strengthening the O—H bond and making \(H^+\) less likely to be donated.
Explanation: The inductive effect of alkyl groups reduces the acidity of alcohols compared to water.
(c)(i)
The boiling points increase from \(H_2S\) to \(H_2Te\) due to stronger London dispersion forces as molecular size and electron count increase.
Explanation: Larger molecules have more electrons, leading to stronger intermolecular forces and higher boiling points.
(c)(ii)
\(H_2O\) has a much higher boiling point than \(H_2S\) due to hydrogen bonding, which is stronger than London dispersion forces.
Explanation: The high electronegativity of oxygen enables hydrogen bonding in \(H_2O\), requiring more energy to break these bonds.
