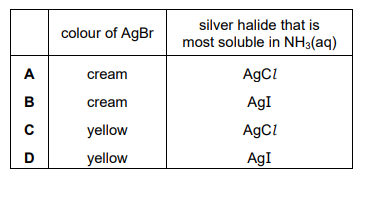
The colours of the silver halides AgCl, AgBr, and AgI differ. The solubilities of these halides in aqueous ammonia also differ. Which row is correct?
▶️ Answer/Explanation
Ans: A
1. Colours: – AgCl is white, – AgBr is cream, – AgI is yellow.
2. Solubility in NH3(aq): – AgCl dissolves in dilute NH3, – AgBr dissolves only in concentrated NH3, – AgI is insoluble in NH3(aq).
Thus, the correct row is A, matching the observed properties of silver halides.
NH₄Cl reacts with NaOH in an aqueous solution. Which statement is correct?
▶️ Answer/Explanation
Ans: A
The reaction between NH₄Cl and NaOH is:
\[ \text{NH}_4\text{Cl} + \text{NaOH} \rightarrow \text{NH}_3 + \text{H}_2\text{O} + \text{NaCl} \]
Analysis of options:
- Option A (Correct): The products NH₃ (polar) and H₂O (polar) are two different polar molecules.
- Option B (Incorrect): The bond angle changes from ~109.5° in NH₄⁺ (tetrahedral) to ~107° in NH₃ (trigonal pyramidal).
- Option C (Incorrect): NH₄⁺ acts as an acid (donates H⁺), not a base.
- Option D (Incorrect): The oxidation state of nitrogen remains −3 in both NH₄⁺ and NH₃.
Thus, Option A is the correct statement.
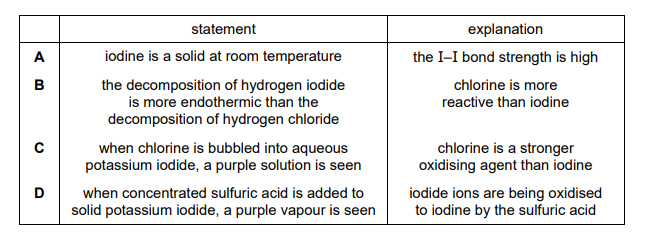
The table shows statements about some of the properties of halogens and their compounds and explanations for these properties. Which row shows a correct statement about the property and a correct explanation for the statement?
▶️ Answer/Explanation
Ans: D
Analysis of the options:
- Option A is incorrect because while the boiling point of halogens increases down the group, the explanation given (stronger van der Waals forces due to more electrons) is correct, but the statement itself is not fully accurate.
- Option B is incorrect because the reactivity of halogens decreases down the group, not increases.
- Option C is incorrect because while the bond enthalpy decreases down the group, the explanation (weaker bonds due to larger atomic size) is correct, but the statement is not.
- Option D is correct because halogenoalkanes with larger halogens (e.g., iodine) undergo nucleophilic substitution reactions more readily due to the weaker carbon-halogen bond, which is correctly explained by the decreasing bond strength down the group.
Thus, Option D is the only row with both a correct statement and a correct explanation.
Chlorine gas is reacted with cold aqueous sodium hydroxide. Which statement is correct for this reaction?
A. Chlorine is both oxidised and reduced.
B. Chlorine is neither oxidised nor reduced.
C. Chlorine is oxidised but not reduced.
D. Chlorine is reduced but not oxidised.
▶️ Answer/Explanation
Ans: A
When chlorine gas (\( Cl_2 \)) reacts with cold aqueous sodium hydroxide (\( NaOH \)), a disproportionation reaction occurs. One chlorine atom is oxidised to \( ClO^- \) (oxidation state changes from 0 to +1), while another is reduced to \( Cl^- \) (oxidation state changes from 0 to -1). Thus, chlorine is both oxidised and reduced, making option A correct.
