Nitrogen dioxide is a gas that contributes to air pollution. It is produced in internal combustion engines. Which statement is correct?
▶️ Answer/Explanation
Ans: A
Nitrogen dioxide (\(NO_2\)) is known to catalyze the oxidation of sulfur dioxide (\(SO_2\)) to sulfur trioxide (\(SO_3\)) in the atmosphere, which is a key step in acid rain formation. This makes statement A correct. The other options are incorrect because:
- B: In catalytic converters, \(NO_2\) is reduced to \(N_2\), not \(NO\).
- C: PAN (Peroxyacyl nitrates) form from \(NO_2\) and incomplete combustion products (hydrocarbons), not complete combustion.
- D: \(NO_2\) forms from \(N_2\) and \(O_2\) under high-temperature combustion, not from fuel impurities.
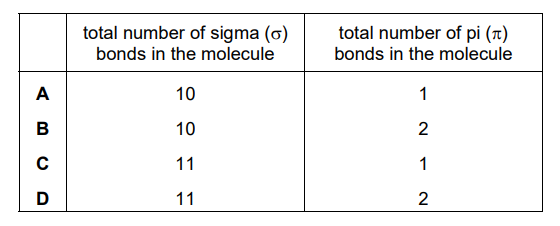
Which row shows the correct number of covalent bonds in a molecule of methylpropene?
▶️ Answer/Explanation
Ans: C
Methylpropene (\(C_4H_8\)) has a double bond between two carbons and single bonds elsewhere. The total number of covalent bonds is 11 (4 C-C bonds and 7 C-H bonds). The correct row in the table is C, which matches this count.
Structural and stereoisomerism should be considered when answering this question. P has molecular formula \(C_5H_{10}O\). P produces an orange precipitate with 2,4-dinitrophenylhydrazine (2,4-DNPH reagent). How many isomeric structures does P have?
A. 5
B. 6
C. 7
D. 8
▶️ Answer/Explanation
Ans: D
The positive test with 2,4-DNPH indicates that P is an aldehyde or ketone (a carbonyl compound with the general formula \(C_5H_{10}O\)). The isomers include pentanal, 2-methylbutanal, 3-methylbutanal, and pentan-2-one, pentan-3-one. Considering structural isomers and stereoisomers: pentanal (1), 2-methylbutanal (1), 3-methylbutanal (1), pentan-2-one (0 stereoisomers), and pentan-3-one (which has a chiral center, giving 2 enantiomers). This totals 6 structural isomers, but pentan-3-one’s stereoisomers bring the final count to 8.
Which two formulae correctly represent a pair of structural isomers?
A. CH₃CH(CH₃)COOH and (CH₃)₂CHCOOH
B. CH₃CH(COOH)CH₃ and (CH₃)₂CHCOOH
C. CH₃CHCOOH and CH₃CH₂CH₂COOH
D. CH₃CH₂CH₂COOH and (CH₃)₂CHCOOH
▶️ Answer/Explanation
Ans: D
Structural isomers are compounds with the same molecular formula but different structural formulae. Option D features two carboxylic acids with the molecular formula \(C_4H_8O_2\): butanoic acid (\(CH_3CH_2CH_2COOH\)) and 2-methylpropanoic acid (\((CH_3)_2CHCOOH\)). They have different carbon skeletons, making them structural isomers.
