Question
The following compounds were all found to be components of a sample of petrol.
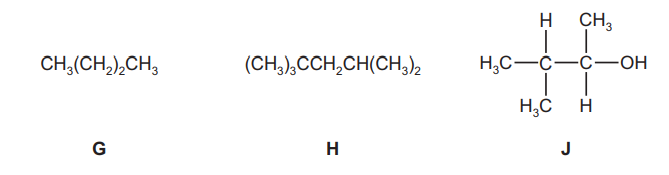
(a) (i) Give the molecular formula of compound G.
……………………………………………………………………………………………………………………… [1]
(ii) Give the empirical formula of compound H.
……………………………………………………………………………………………………………………… [1]
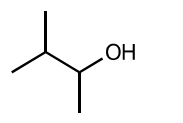
(iii) Draw the skeletal formula of compound J.[1]
(b) Write an equation to represent the complete combustion of compound H.
……………………………………………………………………………………………………………………………. [1]
(c) Fossil fuels are often contaminated with sulfur.
State and explain why supplies of fossil fuels that contain sulfur pose a problem to the environment.
(d) The boiling points of compounds G, H and J are shown below.
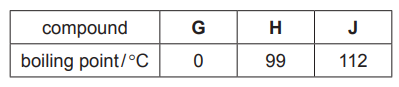
Explain the differences in the boiling points of the three compounds.
(e) Compound $\mathbf{J}$ can be produced from 2-chloro-3-methylbutane, $\mathrm{C}_5 \mathrm{H}_{11} \mathrm{Cl}$.
Give the reagent(s) and conditions for this reaction.[1] [Total: 11]
▶️Answer/Explanation
Ans:
(a) (i) $\underline{\mathrm{C}}_4 \underline{H}_{10}$
(ii) $\quad \underline{\mathrm{C}}_4 \mathrm{H}_9$
(iii)
(b) $\quad \mathrm{C}_8 \mathrm{H}_{18}+12 \frac{1}{2} \mathrm{O}_2 \rightarrow 8 \mathrm{CO}_2+9 \mathrm{H}_2 \mathrm{O}$
(c) sulfur dioxide would be produced on combustion
(which contributes to) acid rain
(d) M1 = H has more/ greater/ stronger van der Waals’/ intermolecular forces than G / ora
M2 = (because)
H has more electrons (thanG)
M3= J has hydrogen bonding (between molecules)
M4 = strong(er)/ great(er) forces require AND high/ more energy to overcome
(e) NaOH(aq)
