Question
Ibuprofen and paracetamol are pain-relief drugs.
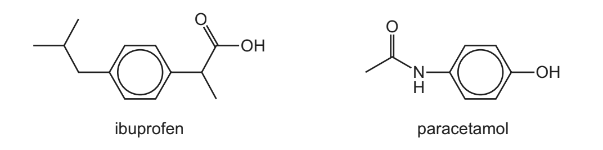
(a) Ibuprofen and paracetamol both contain the aryl (benzene) functional group.
Name the other functional groups present in each molecule.
(b) Ibuprofen contains a chiral centre and shows stereoisomerism.
(i) State what is meant by the term chiral centre.
(ii) Draw the two stereoisomers of ibuprofen.

(c) Draw the structures of the organic products when ibuprofen and paracetamol react separately
with LiAlH4.
(d) A student carried out some reactions with solutions of ibuprofen and paracetamol using
reagents D and E and the following results were obtained.
(✓ means a reaction took place.)
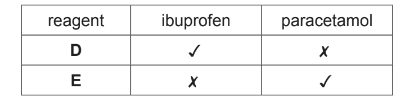
(i) Suggest a possible identity for each reagent D and E.
(ii) Give the structure of the organic product formed when reagent D reacted with ibuprofen.
(iii) Give the structure of the organic product formed when reagent E reacted with paracetamol.
(e) One of the steps in the manufacture of ibuprofen is shown.
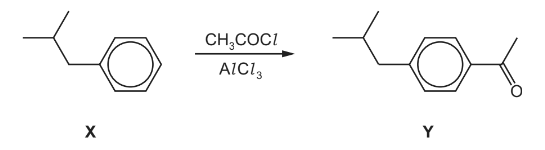
(i) Write an equation for the reaction between CH3COCl and AlCl3.
(ii) Complete the mechanism for the conversion of X into Y. Include all necessary curly arrows,
any relevant dipoles and charges.
(iii) Name the mechanism in (ii).
Answer/Explanation
Answer: (a) ibuprofen: carboxylic acid / carboxyl
paracetamol: phenol and amide
any two = 1 mark
all three = 2 marks
(b)(i) (chiral centre is a) carbon OR atom that has four different groups /atoms / species attached to it
(b)(ii) 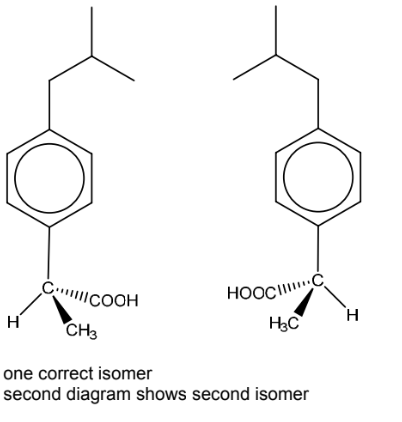
(c) 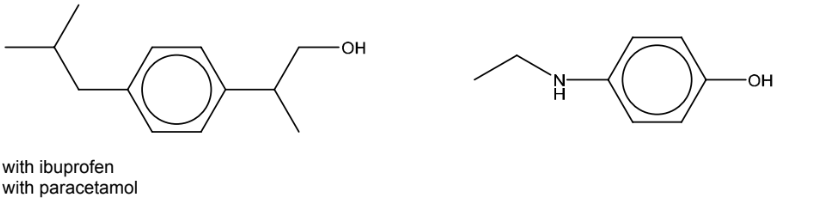
(d)(i) (reagent D) Na2CO3 / any carbonate
(reagentE) Cl2 /Br2
(d)(ii) 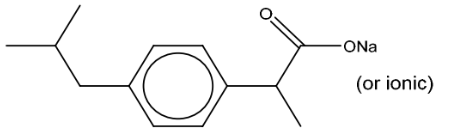
(d)(iii) 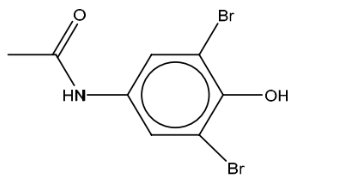
(e)(i) CH3COCl + AlCl3→CH3CO+ + AlCl4–
(e)(ii) 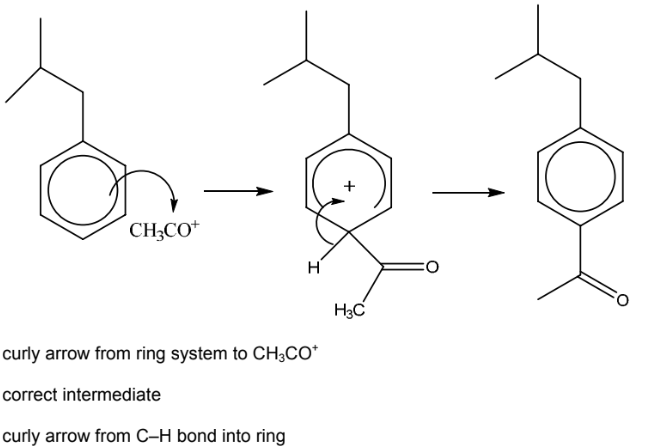
6(e)(iii) electrophilic substitution
