The structure of limonene is shown.
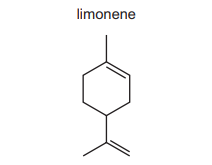
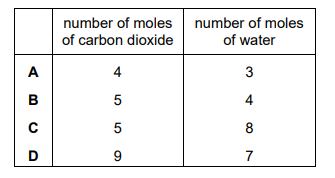
What are the number of moles of carbon dioxide and water produced when a sample of limonene is completely combusted in oxygen?
▶️ Answer/Explanation
Ans: B
Limonene has the molecular formula \( \text{C}_{10}\text{H}_{16} \). The balanced combustion equation is: \[ \text{C}_{10}\text{H}_{16} + 14\text{O}_2 \rightarrow 10\text{CO}_2 + 8\text{H}_2\text{O} \] For 1 mole of limonene, combustion produces: – 10 moles of CO₂ (from 10 carbon atoms). – 8 moles of H₂O (from 16 hydrogen atoms). Thus, option B (10, 8) is correct.
Vitamin A contains retinol.
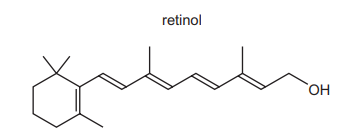
Under appropriate conditions, acidified KMnO₄ (aq) can be used to break C=C bonds. After these bonds have been broken, further oxidation of the fragments may occur. Under which conditions is the acidified KMnO₄ (aq) used and what do the final oxidation products include? 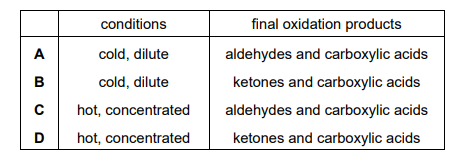
▶️ Answer/Explanation
Ans: D
When hot, concentrated, acidified KMnO4 is used, it cleaves C=C bonds and oxidizes the fragments further. For retinol (with multiple C=C bonds), this leads to the formation of ketones and carboxylic acids (option D). The conditions are harsh (hot and concentrated) to ensure complete oxidative cleavage.
The alkane CH₃CH₂CH(CH₃)₂ undergoes free radical substitution with chlorine. No C–C bonds are broken in this reaction. How many isomeric products, including positional and optical isomers, of molecular formula \(C_5H_{11}Cl\) can be formed?
▶️ Answer/Explanation
Ans: C
1. Structural Isomers: Chlorination can occur at 4 distinct positions (C1, C2, C3, and C4) of the alkane \(CH_3CH_2CH(CH_3)_2\).
2. Stereoisomers: Substitution at C3 (the chiral center) produces a pair of enantiomers (optical isomers).
3. Total Isomers: 4 structural isomers + 1 pair of enantiomers = 6 isomeric products.
Thus, the correct answer is C (6).
Which statement describes a property of an ammonium ion?
▶️ Answer/Explanation
Ans: D
The ammonium ion (\(NH_4^+\)) has a tetrahedral shape with four identical N–H bonds due to sp³ hybridization, making option D correct. Option A is incorrect because the ammonium ion acts as a weak acid (donates \(H^+\)), not a base. Option B is wrong as ammonium sulfate does not react with HCl to form \(NH_3\). Option C describes ammonia (\(NH_3\)), not the ammonium ion, which has a bond angle of ~109.5°.
