Hydrocarbon molecules contain covalent bonds.
(a) Define covalent bond.
(b) A C=C bond in an alkene is made from a σ bond and a π bond.
(i) Identify the hybridisation of the carbon atoms in a C=C bond in an alkene.
(ii) Draw labelled diagrams to show, in terms of orbital overlap, how the σ and π bonds are made in a C=C bond.
σ bond
π bond
(c) In electrophilic reactions involving alkenes the π bond of C=C is broken.
(i) Suggest one difference between σ and π bonds that explains why the π bond is broken in electrophilic addition reactions involving alkenes.
(ii) Complete Fig. 5.1 to show the mechanism for the electrophilic addition of hydrogen bromide to 2-methylpropene to produce the major organic product. Include charges, dipoles, lone pairs of electrons and curly arrows, as appropriate.
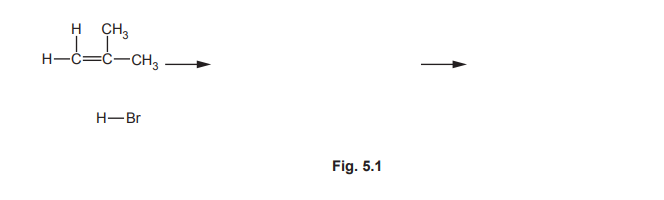
▶️ Answer/Explanation
(a) (electrostatic) attraction between nuclei of two atoms and shared pair of electrons
Explanation: A covalent bond is formed when two atoms share a pair of electrons, resulting in an electrostatic attraction between the positively charged nuclei and the negatively charged shared electrons.
(b)(i) \(sp^2\)
Explanation: In a C=C bond, each carbon atom forms three σ bonds (two with hydrogen and one with the other carbon) using \(sp^2\) hybrid orbitals, leaving one unhybridized p orbital for the π bond.
(b)(ii)
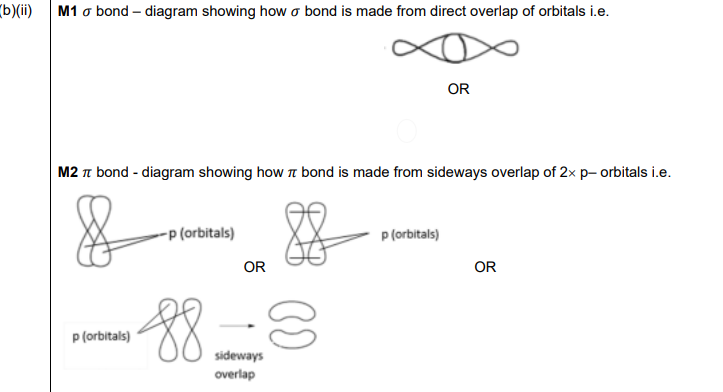
Explanation: The σ bond is formed by head-on overlap of \(sp^2\) hybrid orbitals, while the π bond is formed by sideways overlap of the remaining p orbitals perpendicular to the plane of the σ bonds.
(c)(i) EITHER (pair of) electrons in 𝜋 bond are further away from the nuclei so weaker attraction OR (pair of) electrons in 𝜎 bond are closer to the two nuclei so stronger attraction
Explanation: The π bond is weaker because its electron density is distributed above and below the internuclear axis, making it more accessible to electrophiles compared to the σ bond which has direct overlap along the axis.
(c)(ii)
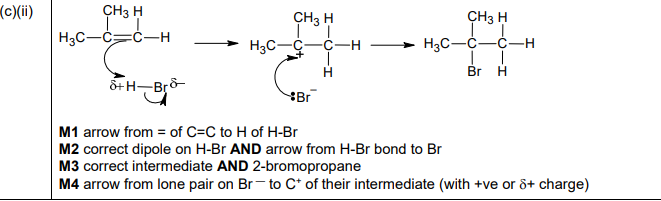
Explanation: The mechanism shows the π electrons attacking HBr, forming a carbocation intermediate on the more substituted carbon (Markovnikov’s rule), followed by bromide ion attack to form 2-bromo-2-methylpropane.
