Compound Z has the molecular formula \(C_4H_8O_2\). Compound Z reacts with propan-1-ol in the presence of concentrated H₂SO₄. The diagram shows the skeletal formulae of three compounds, S, T and U.
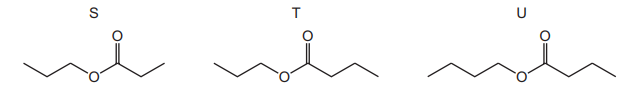
What are the possible skeletal formulae of the products of the reaction between compound Z and propan-1-ol?
▶️ Answer/Explanation
Ans: D
Given that compound Z (\(C_4H_8O_2\)) reacts with propan-1-ol under acidic conditions, the reaction is likely an esterification (forming an ester). The molecular formula \(C_4H_8O_2\) suggests Z is a carboxylic acid (e.g., butanoic acid or its isomers). The product of this reaction with propan-1-ol would be an ester (propyl butanoate or its isomers). Comparing the skeletal structures:
- S represents a different functional group (not an ester).
- T matches the structure of propyl butanoate (ester).
- U is not an ester and does not fit the expected product.
Thus, the only correct product is T, making option D correct.
What is true of every nucleophile?
▶️ Answer/Explanation
Ans: B
A nucleophile (“nucleus-loving”) is defined by its ability to donate a lone pair of electrons to an electrophile. While nucleophiles often attack electrophilic centers (including some double bonds, A), this is not universal. They can be neutral (e.g., \(NH_3\)) or molecular (e.g., \(H_2O\)), ruling out C and D. Thus, B is the only universally true statement.
Which compound can undergo nucleophilic addition?
▶️ Answer/Explanation
Ans: B
Ethanal (CH₃CHO) is the only compound among the options that can undergo nucleophilic addition due to its polar carbonyl group (C=O). The electrophilic carbon in the carbonyl group attracts nucleophiles, leading to addition reactions. For example, with NaBH₄ or HCN:
\[ CH_3CHO + HCN \rightarrow CH_3CH(OH)CN \]
Why other options cannot undergo nucleophilic addition:
- A (C₂H₅Br): Undergoes nucleophilic substitution (not addition).
- C (C₂H₆): Saturated hydrocarbon; no functional group to attract nucleophiles.
- D (C₂H₄): Undergoes electrophilic addition (e.g., with Br₂), not nucleophilic addition.
The reaction of chlorine with methane is carried out in the presence of light. What is the function of the light?
▶️ Answer/Explanation
Ans: B
In the free-radical substitution reaction between chlorine and methane, light plays a crucial role:
- Initiation step: Light provides energy to break the Cl–Cl bond homolytically, forming highly reactive chlorine radicals (Cl•).
- Why not other options?:
- A: C–H bonds in methane are not directly broken by light.
- C: Light does not ionize chlorine (no ions are formed).
- D: While heat is generated, the primary role of light is to initiate the reaction by generating radicals.
Thus, the correct function of light is to break chlorine molecules into atoms (Option B).
