Bromoalkanes are used widely in industry, although there is increasing concern about their environmental impact.
(a) Complete Fig. 4.2 to show the mechanism for the formation of 1,2-dibromoethane in reaction 1. Include charges, dipoles, lone pairs of electrons and curly arrows as appropriate. 
(b) The enthalpy change of reaction 1, \(\Delta H_r\) = –90.0kJmol⁻¹.
The enthalpy change of formation of ethene, \(ΔH_f\) = +52.2 kJ mol⁻¹. Calculate the enthalpy change of formation of 1,2-dibromoethane.
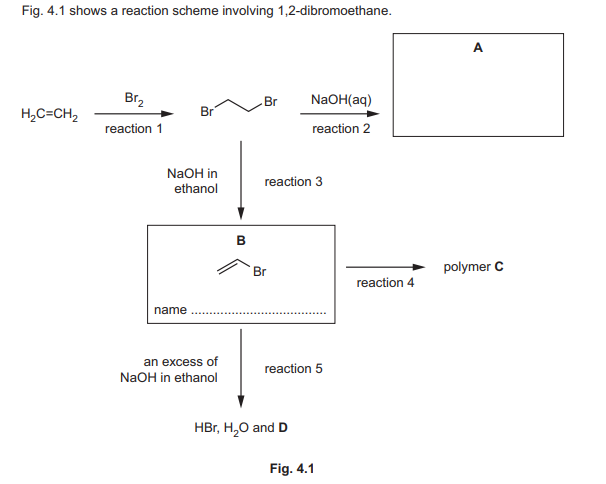
(c) (i) Complete Fig. 4.1 to:
• draw the structure of compound A
• name compound B.
(ii) Draw the structure of one repeat unit of polymer C in the box.

(iii) In reaction 5, compound B reacts with an excess of NaOH dissolved in ethanol. The products are HBr, \(H_2O\), and an unsaturated hydrocarbon D. Suggest the identity of D.
(d) Compound E is the only isomer of 1,2-dibromoethane. Alkaline hydrolysis of E gives compound F.
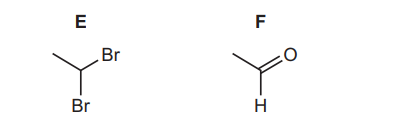
(i) Identify the type of isomerism shown by E and 1,2-dibromoethane.
(ii) Name the homologous series that F belongs to.
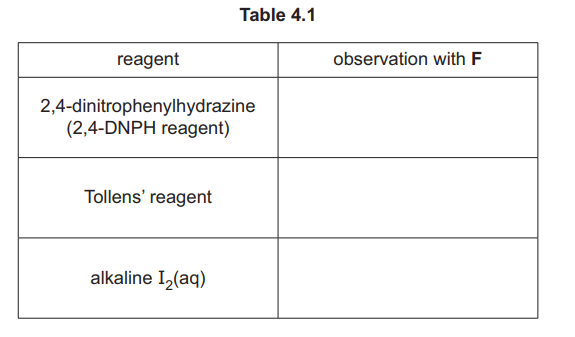
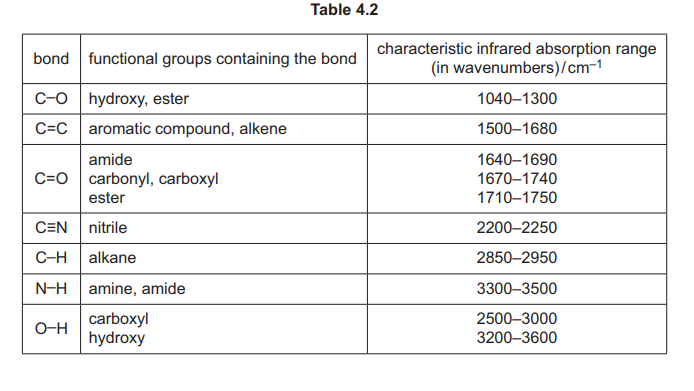
(iii) Complete Table 4.1 to state what is observed when F reacts with the reagents listed.
(e) Compound F reacts with reagent G to form compound H.
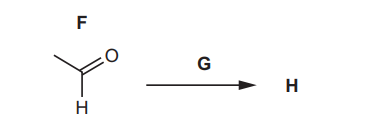
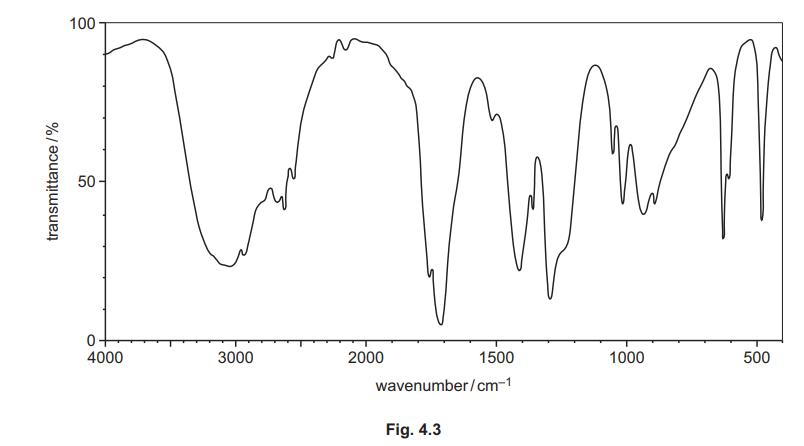
The infrared spectrum of H is shown in Fig. 4.3.
H also shows a molecular ion peak at m/e = 60 in its mass spectrum.
(i) Use the information in (e), Fig. 4.3 and Table 4.2 to deduce the structure of H. Explain your answer fully. 
(ii) Suggest the role of reagent G.
▶️ Answer/Explanation
(a)
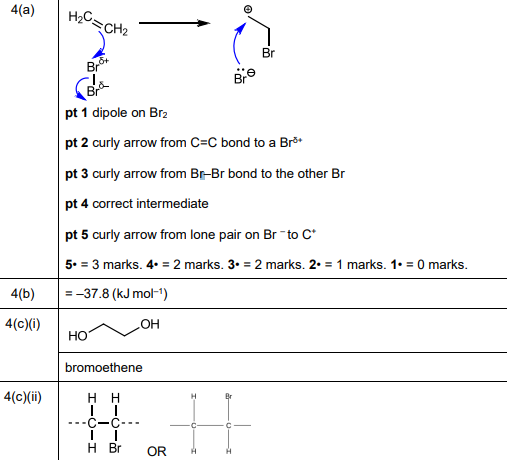
Explanation: The mechanism involves electrophilic addition where the π bond in ethene attacks Br₂, forming a bromonium ion intermediate. The Br⁻ then attacks from the opposite side, resulting in 1,2-dibromoethane.
(b) \(\Delta H_f\) of 1,2-dibromoethane = –142.2 kJ mol⁻¹.
Explanation: Using Hess’s Law, \(\Delta H_r = \Delta H_f(\text{products}) – \Delta H_f(\text{reactants})\). Given \(\Delta H_r = -90.0\) kJ mol⁻¹ and \(\Delta H_f(\text{ethene}) = +52.2\) kJ mol⁻¹, solving gives \(\Delta H_f(\text{1,2-dibromoethane}) = -90.0 – 52.2 = -142.2\) kJ mol⁻¹.
(c)(i) A: \(CH_2BrCH_2Br\), B: 1,2-dibromoethane.
(ii) Polymer C repeat unit: \(-CH_2-CH_2-\)
(iii) D: \(C_2H_2\) (ethyne).
Explanation: Elimination of HBr from 1,2-dibromoethane in the presence of NaOH/ethanol produces ethyne.
(d)(i) Structural/positional isomerism.
(ii) Aldehyde.
(iii)
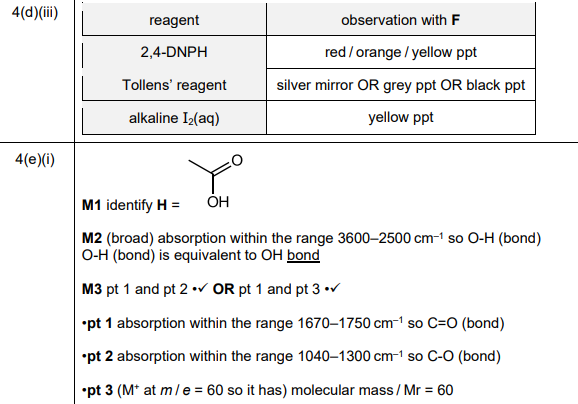
Explanation: F (ethanal) forms a silver mirror with Tollens’ reagent and a red precipitate with Fehling’s solution due to its aldehyde group.
(e)(i) H: \(CH_3COOH\) (ethanoic acid).
Explanation: The IR spectrum shows a broad O-H stretch (~3000 cm⁻¹) and a C=O stretch (~1700 cm⁻¹), consistent with a carboxylic acid. The mass spectrum peak at m/e = 60 matches ethanoic acid’s molecular weight.
(ii) Oxidising agent.
Explanation: Reagent G (e.g., acidified KMnO₄ or K₂Cr₂O₇) oxidises the aldehyde (F) to a carboxylic acid (H).
