Question
A series of reactions based on propanoic acid is shown
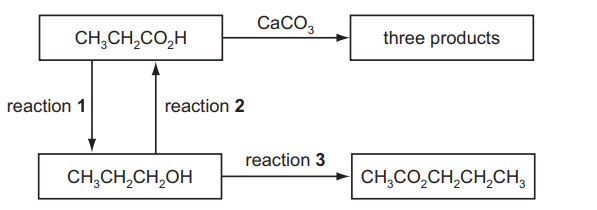
(a) Write an equation for reaction $\mathbf{1}$, using $[\mathrm{H}]$ to represent the reducing agent.[2]
(b) (i) What type of reaction is reaction 2?
(ii) Suggest a suitable reagent and conditions for reaction 2.[2]
(c) Write an equation for the reaction of propanoic acid with calcium carbonate, $\mathrm{CaCO}_3$.[2]
(d) (i) Suggest a suitable reagent and conditions for reaction 3.[2]
(ii) Identify the other product of reaction 3 .[1][Total: 10]
▶️Answer/Explanation
Ans:
(a) $\quad \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}+4[\mathrm{H}] \rightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}+\mathrm{H}_2 \mathrm{O}$
(b) (i) Oxidation
(ii) Sodium/potassium dichromate or correct formula $\mathrm{H}^{+} /$acidified and (heat under) reflux
(c) $\quad 2 \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2 \mathrm{H}+\mathrm{CaCO}_3 \rightarrow\left(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CO}_2\right)_2 \mathrm{Ca}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2$
(d) (i) $\mathrm{CH}_3 \mathrm{CO}_2 \mathrm{H}$
warm/hot/high temperature/heat/reflux AND concentrated sulfuric acid
(ii) water (or hydrogen chloride or ethanoic acid)
