Propanone, CH₃COCH₃, is an important organic reagent. Fig. 4.1 shows some reactions of propanone and its derivatives.
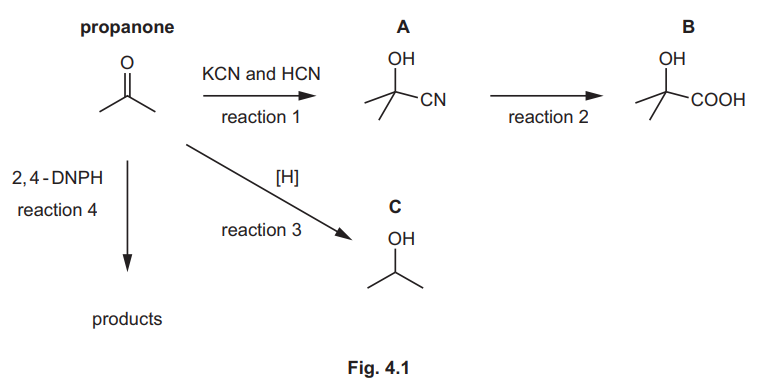
(a) Reaction 1 is a nucleophilic addition reaction.
(i) Complete Fig. 4.2 to show the mechanism for the formation of A from propanone. Include charges, dipoles, lone pairs of electrons and curly arrows as appropriate. 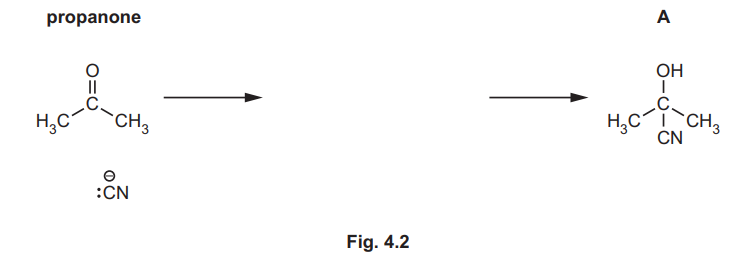
(ii) Explain why A does not show optical isomerism.
(b) Suggest the reagents and conditions for reaction 2.
(c) Reaction 3 is a reduction reaction.
(i) Construct an equation to represent reaction 3. Use [H] to represent one atom of hydrogen from the reducing agent.
(ii) Name C.
(d) State what is observed in reaction 4.
(e) Explain why Fehling’s reagent does not react with propanone.
(f) Compounds A, B and C can be distinguished using infrared spectroscopy.
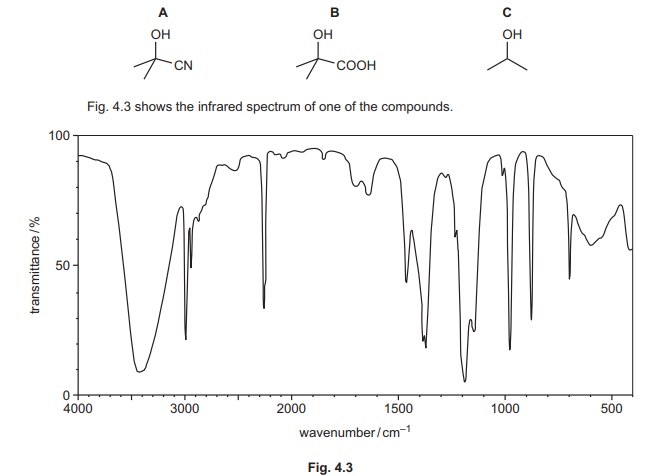
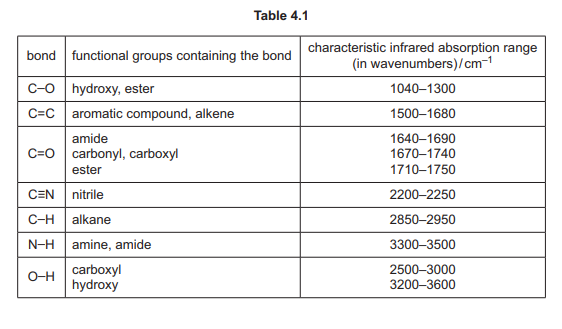
(i) Explain why the absorptions at 2850–2950 cm⁻¹ are not useful to help determine which of the compounds A, B or C produces the infrared spectrum in Fig. 4.3. Use Table 4.1 to answer this question.
(ii) Identify which of compounds A, B or C produces the infrared spectrum in Fig. 4.3. Explain your answer.
▶️ Answer/Explanation
(a)(i) The mechanism for the nucleophilic addition of HCN to propanone is shown below:
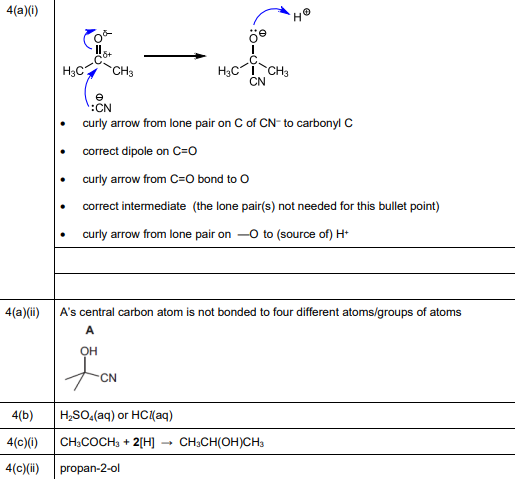
Explanation: The nucleophilic cyanide ion attacks the electrophilic carbonyl carbon, followed by protonation to form the cyanohydrin (A).
(a)(ii) A does not show optical isomerism because it lacks a chiral center.
Explanation: The product (2-hydroxy-2-methylpropanenitrile) has no asymmetric carbon, as all groups attached to the central carbon are different but not arranged in a way that creates non-superimposable mirror images.
(b) Reagents: K₂Cr₂O₇/H₂SO₄ (acidified potassium dichromate). Conditions: Heat under reflux.
Explanation: This oxidizes the secondary alcohol (B) to propanone.
(c)(i) Equation: \(CH_3COCH_3 + 4[H] \rightarrow CH_3CH(OH)CH_3\).
Explanation: Propanone is reduced to propan-2-ol (C) using a reducing agent like NaBH₄ or LiAlH₄.
(c)(ii) C is propan-2-ol.
Explanation: The reduction of propanone yields a secondary alcohol.
(d) A red/orange/yellow precipitate forms.
Explanation: Reaction with 2,4-DNPH (Brady’s reagent) produces a colored hydrazone derivative.
(e) Fehling’s reagent does not oxidize ketones.
Explanation: Fehling’s solution (alkaline Cu²⁺) oxidizes aldehydes but not ketones, as ketones lack an easily oxidizable aldehyde hydrogen.
(f)(i) Absorptions at 2850–2950 cm⁻¹ (C-H stretches) are common to all organic compounds.
Explanation: Since A, B, and C all contain C-H bonds, this region cannot distinguish between them.
(f)(ii) Compound A (2-hydroxy-2-methylpropanenitrile).
Explanation: The sharp peak at 2200–2250 cm⁻¹ (C≡N stretch) is unique to A, as B and C lack nitrile groups.
