Question
Which statement is correct?
A $\mathrm{C} l$ has a relative isotopic mass of 35.5 .
B $\mathrm{Cl}_2$ has a relative molecular mass of 70 .
C IC $l$ has a relative molecular mass of 162.4 .
D $\mathrm{NaC} l$ has a relative molecular mass of 58.5 .
▶️Answer/Explanation
Ans:C
Question
6.90 g of an ammonium salt is heated with an excess of aqueous sodium hydroxide. The volume
of ammonia produced, measured under room conditions, is \(2.51dm^{3}\)
Which ammonium salt is used?
A ammonium carbonate \(M_{r}=96.0\)
B ammonium chloride \(M_{r}=53.5\)
C ammonium nitrate \(M_{r}=80.0\)
D ammonium sulfate \(M_{r}=132.1\)
▶️Answer/Explanation
Ans:D
Question
Which statement about atoms and molecules is correct?
A The molecular formula of a compound is the simplest whole number ratio of atoms of each element in the compound.
B One mole of any substance contains \(6 times 10^{23}\) atoms.
C The relative atomic mass of an element is the ratio of the average mass of one atom of the element to the mass of an atom of carbon-12.
D The relative formula mass of a compound is the sum of the individual atomic masses of all the atoms in the formula.
Answer/Explanation
Ans: D
Question
A butane burner is used to heat water. The Mr of butane is 58.
● ΔHθc of butane is –2877kJ mol–1.
● 250 g of water is heated from 12 °C to 100 °C.
● The burner transfers 47% of the heat released from the burning fuel to the water.
Assume that the butane undergoes complete combustion and none of the water evaporates.
What is the minimum mass of butane that must be burnt?
A 0.068 g B 1.85 g C 3.94 g D 4.48 g
Answer/Explanation
Answer C
Question
A sample of the hydrocarbon C6H12 is completely burned in dry oxygen and the product gases arencollected as shown.
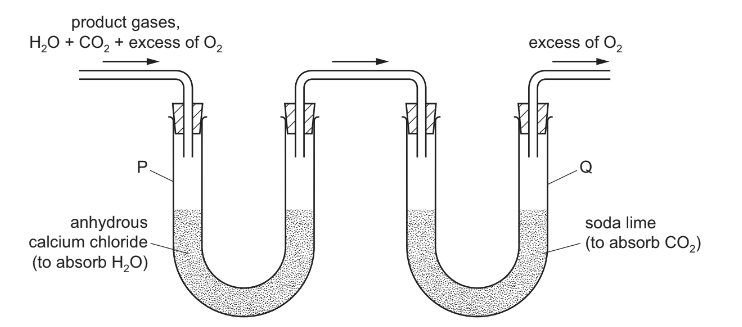
The increases in mass of the collecting vessels P and Q are MP and MQ, respectively.
What is the ratio MP / MQ?
A 0.41 B 0.82 C 1.2 D 2.4
Answer/Explanation
Answer A
Question
The mass spectrum of a sample of lithium shows that it contains two isotopes, \(^6Li\) and\(^7Li\).
The isotopic abundances are shown in the table.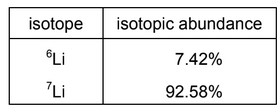
What is the relative atomic mass of this sample of lithium, given to three significant figures?
A 6.07 B 6.50 C 6.90 D 6.93
Answer/Explanation
Ans: D
Question
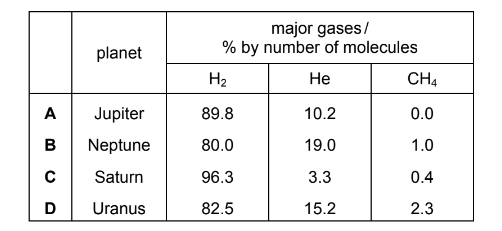
The approximate percentage composition of the atmospheres on four different planets is given in the table below.
Which mixture of gases has the greatest density?
Answer/Explanation
Answer: D
Question
Tetraethyl lead, Pb(C2H5)4, has been used as a petrol additive.
What is the percentage by mass of carbon in tetraethyl lead?
A 10.2 B 14.9 C 29.7 D 32.0
Answer/Explanation
Answer: C
Question
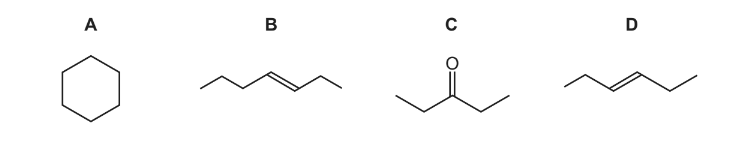
Use of the Data Booklet is relevant to this question. Which compound has an Mr of 84 and will react with HBr to give a product with an Mr of 164.9?
Answer/Explanation
Ans:D
Question
Use of the Data Booklet is relevant to this question.
2.30g of ethanol were mixed with an excess of aqueous acidified potassium dichromate(VI). The reaction mixture was then boiled under reflux for one hour. The desired organic product was then collected by distillation. The yield of product was 60.0%.
What mass of product was collected?
A 1.32g B 1.38g C 1.80g D 3.20g
Answer/Explanation
Ans: C
Question
Use of the Data Booklet is relevant to this question.
A sample of ethyl propanoate is hydrolysed by heating under reflux with aqueous sodium hydroxide. The two organic products of the hydrolysis are separated, purified and weighed.
Out of the total mass of products obtained, what is the percentage by mass of each product?
- 32.4% and 67.6%
- 38.3% and 61.7%
- 42.3% and 57.7%
- 50.0% and 50.0%
Answer/Explanation
Ans:
A
Question
Use of the Data Booklet is relevant to this question.
Which sodium compound contains 74.2% by mass of sodium?
A sodium carbonate
B sodium chloride
C sodium hydroxide
D sodium oxide
Answer/Explanation
Ans: D
Question
Use of the Data Booklet is relevant to this question.
The approximate percentage composition of the atmosphere on four different planets is given in the table below.
The density of a gas may be defined as the mass of \(1dm^3\) of the gas measured at s.t.p.
Which mixture of gases has the greatest density?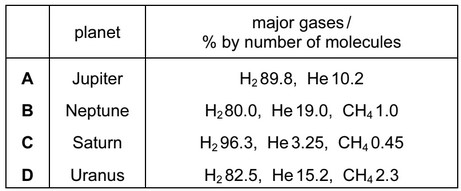
Answer/Explanation
Ans: D
