Question
A sample of solid ammonium chloride decomposes on heating.
solid ammonium chloride → ammonia gas + hydrogen chloride gas
A total of \(2.4~x~10^{21}\) molecules of gas is formed.
How many hydrogen atoms are present in the gaseous products?
A \(1.2~x~10^{21}\) B \(2.4~x~10^{21}\) C \(4.8~x~10^{21} \) D \( 9.6~x~10^{21}\)
▶️Answer/Explanation
Ans:C
Question
Two moles of compound $\mathrm{P}$ were placed in a sealed container. The container was heated and $\mathrm{P}$ was partially decomposed to produce $\mathrm{Q}$ and $\mathrm{R}$ only. A dynamic equilibrium between $\mathrm{P}, \mathrm{Q}$ and $\mathrm{R}$ was established.
At equilibrium $x$ moles of $R$ were present and the total number of moles present was $\left(2+\frac{x}{2}\right)$.
What is the equation for this reversible reaction?
$A P \rightleftharpoons 2 Q+R$
B $2 \mathrm{P} \rightleftharpoons 2 \mathrm{Q}+\mathrm{R}$
C $2 \mathrm{P} \rightleftharpoons \mathrm{Q}+\mathrm{R}$
D $2 P \rightleftharpoons Q+2 R$
▶️Answer/Explanation
Ans:D
Question
Which statement about atoms and molecules is correct?
A The molecular formula of a compound is the simplest whole number ratio of atoms of each element in the compound.
B One mole of any substance contains \(6 times 10^{23}\) atoms.
C The relative atomic mass of an element is the ratio of the average mass of one atom of the element to the mass of an atom of carbon-12.
D The relative formula mass of a compound is the sum of the individual atomic masses of all the atoms in the formula.
Answer/Explanation
Ans: D
Question
‘Black powder’ is a mixture of potassium nitrate, carbon and sulfur. The mixture reacts as shown.
\(4KNO_3(s) + 7C(s) + S(s) \rightarrow 3CO_2(g) + 3CO(g) + 2N_2(g) + K_2S(s) + K_2CO_3(s)\)
A sealed tube containing black powder has a volume of 10.0 \(cm^3\). When all of the black powder reacts, the reaction causes a pressure of \(2 \times 10^6\)Pa and a temperature of 2500K.
The volume of the \(K_2CO_3\) and \(K_2S\) produced can be ignored.
How many moles of \(KNO_3\) are contained in the sealed tube?
A \(4.81 \times 10^{–4}\) B \(9.63 \times 10^{–4}\) C \(1.93 \times 10^{–3}\) D \(9.63 \times 10^{–1}\)
Answer/Explanation
Ans: A
Question
Which contains the largest number of hydrogen atoms?
A 0.10 mol of pentane
B 0.20 mol of but-2-ene
C 1.00 mol of hydrogen molecules
D 6.02 x 1023 hydrogen atoms
Answer/Explanation
Answer: C
Question
A sample of gas occupies 240 cm3 at 37 °C and 100 kPa.
How many moles of gas are present in the sample?
A 9.32 × 10–6 B 9.32 × 10–3 C 0.0781 D 78.1
Answer/Explanation
Answer:
B
Question
Acidified potassium manganate(VII) reacts with iron(II) ethanedioate, FeC2O4.
The reactions taking place are shown.
MnO4– + 8H+ + 5e– → Mn2+ + 4H2O
Fe2+ → Fe3+ + e–
C2O42– → 2CO2 + 2e–
How many moles of iron(II) ethanedioate react with one mole of potassium manganate(VII)?
A 0.60 B 1.67 C 2.50 D 5.00
Answer/Explanation
Answer:
B
Question
Diamond is a pure form of carbon. The mass of a diamond can be measured in carats. One carat is 0.200 g of carbon.
Which expression gives the number of carats that contain 6.02 × 1023 carbon atoms?
A 0.200 × 12.0
B\(\frac{0.200}{12.0}\)
C\(\frac{12.0}{0.200}\)
D \(\frac{0.200}{6.02×10^{23}}×12.0\)
Answer/Explanation
Question
One mole of sulfuric acid is used to make an aqueous solution. The solution contains H2SO4 molecules, H+ ions, SO42– ions and HSO4– ions.
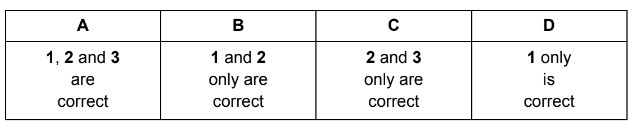
Which statements are correct?
1 The solution contains 6.02 × 1023 sulfur atoms.
2 The solution contains an exactly equal number of H+ ions and HSO4–ions.
3 One mole of SO42– ions contains two moles of electrons.
Answer/Explanation
Answer D
Question
A sports medal has a total surface area of 150 \(cm^2\). It was evenly coated with silver by electrolysis. Its mass increased by 0.216g.
How many atoms of silver were deposited per \(cm^2\) on the surface of the medal?
A \(8.0 × 10^{18}\) B \(1.8 × 10^{19}\) C \(8.7 × 10^{20}\) D \(1.2 × 10^{21}\)
Answer/Explanation
Ans: A
Question
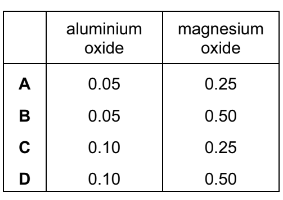
A white powder is known to be a mixture of magnesium oxide and aluminium oxide. 100cm3 of 2 moldm–3 NaOH(aq) is just sufficient to cause the aluminium oxide in x grams of the mixture to dissolve.
The reaction occurring is Al2O3 + 2OH–+ 3H2O → 2Al(OH)4–
800cm3 of 2 moldm–3 HCl(aq) is just sufficient to cause all of the oxide in x grams of the mixture to dissolve.
The reactions occurring are Al2O3 + 6H+→ 2Al3+ + 3H2O and MgO + 2H+→ Mg2+ + H2O.
How many moles of each oxide are present in x grams of the mixture?
Answer/Explanation
Answer: D
Question
The citrus flavour of lemons is due to the compound limonene, present in both the peel and the juice.
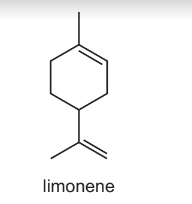
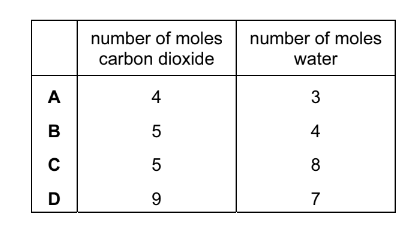
What is the mole ratio of carbon dioxide to water produced when limonene is completely burnt in oxygen?
Answer/Explanation
Ans:B
Question
How many moles of oxygen molecules are needed for the complete combustion of one mole of 3-methylpent-2-ene?
A 9
B \(9\frac{1}{2}\)
C 18
D 19
Answer/Explanation
Ans:A
Question
A solution of \(Sn^{2+}\) ions will reduce an acidified solution of \(MnO_4^–\) ions to \(Mn^{2+}\) ions. The \(Sn^{2+}\) ions are oxidised to \(Sn^{4+}\) ions in this reaction.
How many moles of \(Mn^{2+}\) ions are formed when a solution containing 9.5 g of \(SnCl_2\) (Mr: 190) is added to an excess of acidified \(KMnO_4\) solution?
A 0.010 B 0.020 C 0.050 D 0.125
Answer/Explanation
Ans: B
