Question:
Substance Q is a hydrocarbon. When 1.00 g of Q is completely burned, 3.22 g of carbon dioxide is produced. What could be the identity of Q?
A cyclohexene
B cyclopentane
C ethene
D pentane?
▶️Answer/Explanation
Ans:A
Question:
X and Y are oxides of different Period 3 elements. If one mole of X is added to water, the solution formed is neutralised by exactly one mole of Y. What could be the identities of X and Y?
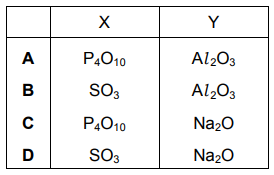
▶️Answer/Explanation
Ans:D
Question
For which hydrocarbon are the molecular and empirical formulae the same?
A butane
B ethane
C pent-1-ene
D propane
▶️Answer/Explanation
ANS:D
Question
Compounds $\mathrm{J}$ and $\mathrm{K}$ each contain $40 \%$ carbon by mass.
What could $\mathrm{J}$ and $\mathrm{K}$ be?
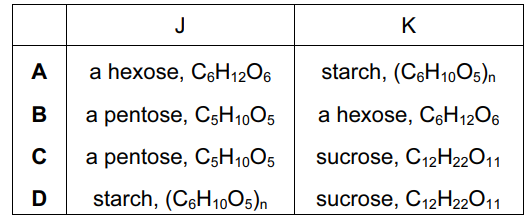
▶️Answer/Explanation
Ans:B
