Question:
Originally, chemists thought indium oxide had the formula InO. By experiment they showed that 4.8 g of indium combined with 1.0 g of oxygen to produce 5.8 g of indium oxide. The Ar of oxygen was known to be 16. Which value for the Ar of indium is calculated using these data?
A 38 B 77 C 115 D 154
▶️Answer/Explanation
Ans:B
Question
Strontium metal can be extracted from strontium oxide, $\mathrm{SrO}$, by reduction with aluminium. One of the possible reactions is shown.
$
6 \mathrm{SrO}+2 \mathrm{Al} \rightarrow 3 \mathrm{Sr}+\mathrm{Sr}_3 \mathrm{Al}_2 \mathrm{O}_6
$
What is the maximum mass of strontium metal that can be produced from the reduction of $100 \mathrm{~g}$ of strontium oxide using this reaction?
A $41.3 \mathrm{~g}$
B $42.3 \mathrm{~g}$
C $84.6 \mathrm{~g}$
D $169.2 \mathrm{~g}$
▶️Answer/Explanation
Ans:B
Question
In an experiment, $0.600 \mathrm{~mol}$ of chlorine gas, $\mathrm{Cl}_2$, is reacted with an excess of hot aqueous sodium hydroxide. One of the products is $\mathrm{NaClO}_3$.
Which mass of $\mathrm{NaClO}_3$ is formed?
A $21.3 \mathrm{~g}$
B $44.7 \mathrm{~g}$
C $\quad 63.9 \mathrm{~g}$
D $128 \mathrm{~g}$
▶️Answer/Explanation
Ans:A
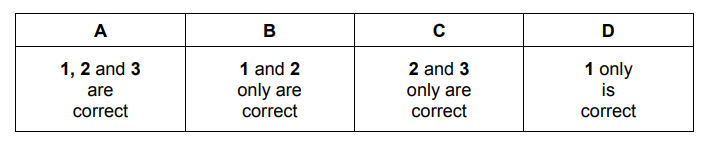
Question
A sample containing $0.40 \mathrm{~mol}$ of calcium nitrate was decomposed by heating in a roaring Bunsen burner flame until there was no further decomposition.
What are the products of this reaction?
$1 \quad 0.40 \mathrm{~mol}$ of calcium oxide
$20.40 \mathrm{~mol}$ of nitrogen, $\mathrm{N}_2(\mathrm{~g})$
$3 \quad 0.40 \mathrm{~mol}$ of oxygen, $\mathrm{O}_2(\mathrm{~g})$
▶️Answer/Explanation
Ans:D
