The diagram shows a section of an addition polymer. The polymer is made using two different monomers.
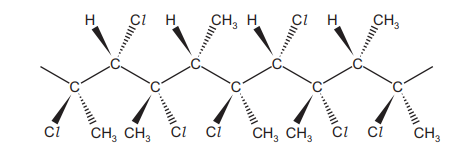
What are the names of the two monomers needed to make this polymer?
A. 1,2-dichloropropene and 2-chlorobut-2-ene
B. 2,3-dichlorobut-2-ene and chloropropene
C. 1,2-dichloropropene and chloroethene
D. chloropropene and 2-chlorobut-2-ene
▶️ Answer/Explanation
Ans: A
1. Analyzing the Polymer Structure: The polymer segment shows repeating units with alternating patterns of chlorine (Cl) substitution. One unit has two Cl atoms on adjacent carbons (1,2-disubstituted), and the other has one Cl on a carbon adjacent to a double bond (2-substituted).
2. Identifying Monomers:
– The 1,2-dichloropropene monomer (\( \text{CH}_2=\text{CCl}-\text{CH}_2\text{Cl} \)) provides the 1,2-dichloro unit.
– The 2-chlorobut-2-ene monomer (\( \text{CH}_3-\text{CCl}=\text{CH}-\text{CH}_3 \)) provides the monosubstituted unit.
3. Eliminating Incorrect Options:
– B and C are incorrect because they either mismatch substitution patterns or introduce incorrect monomers (e.g., chloroethene lacks the required branching).
– D is incorrect because chloropropene alone cannot account for the 1,2-dichloro unit.
4. Conclusion: The correct monomers are 1,2-dichloropropene and 2-chlorobut-2-ene (Option A), as they perfectly match the polymer’s repeating units.
Synthetic resins can be made by polymerisation of a variety of monomers including prop-2-en-1-ol, CH₂=CHCH₂OH. Which structure represents the repeat unit in the polymer poly(prop-2-en-1-ol)?
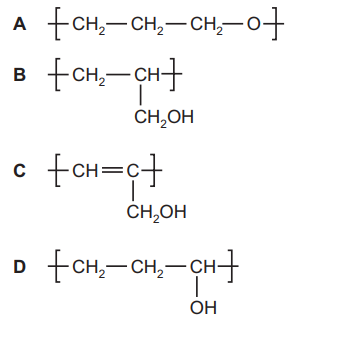
▶️ Answer/Explanation
Ans: B
The repeat unit of poly(prop-2-en-1-ol) is derived from the monomer CH₂=CHCH₂OH through addition polymerization. The double bond breaks to form single bonds with adjacent units, creating the repeat unit −[CH₂−CH(CH₂OH)]−. This matches option B in the image, where the hydroxyl group (−OH) remains attached to the side chain (CH₂) of the main polymer backbone.
Key points:
- The double bond in the monomer opens up to form single bonds in the polymer.
- The hydroxyl group position remains unchanged in the repeat unit.
- Option B correctly shows the −CH₂OH side group attached to every other carbon in the chain.
An addition polymer is made from monomer Z.

What is the structure of the polymer made from this monomer?

▶️ Answer/Explanation
Ans: D
The monomer Z is a substituted alkene that undergoes addition polymerization. The double bond opens up to form the polymer chain, with the substituents (methyl group and COOCH3 group) alternating on every other carbon atom in the polymer backbone.
Key features of the correct polymer structure (Option D):
- The polymer backbone consists of -CH2-CH- repeating units
- Each CH unit has two substituents: a methyl group (CH3) and a methoxycarbonyl group (COOCH3)
- The substituents maintain the same relative positions as in the monomer
This arrangement is characteristic of addition polymers formed from asymmetrically substituted alkenes, where the substituents appear on every other carbon in the polymer chain.
Compound X is treated with an excess of dilute aqueous potassium hydroxide.
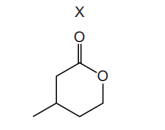
What is the structure of the organic product? 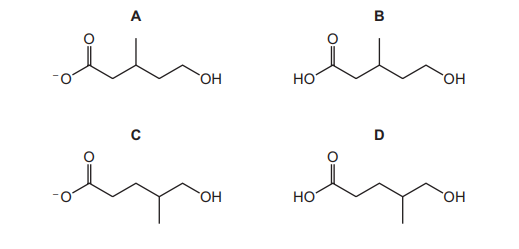
▶️ Answer/Explanation
Ans: A
– Reaction Type: The reaction shown is a nucleophilic substitution where the bromine (Br) in the halogenoalkane is replaced by a hydroxyl group (OH) from KOH. – Product Identification: The product retains the same carbon skeleton but now has an OH group where the Br was originally attached. – Option Analysis: Among the given options, only option A correctly shows the structure with the OH group in the correct position. – Conclusion: The correct organic product is represented by option A.
