Compound W, CH2=CHCN, is used to make an addition polymer which is present in carbon fibres.
(a) Draw one repeat unit of the addition polymer of W.
(b) CH3CHO is used in a two-step synthetic route to form W, as shown in Fig. 6.1. In step 1, CH3CHO is heated with HCN in the presence of KCN.
(i) Name the mechanism for the reaction in step 1 in Fig. 6.1.
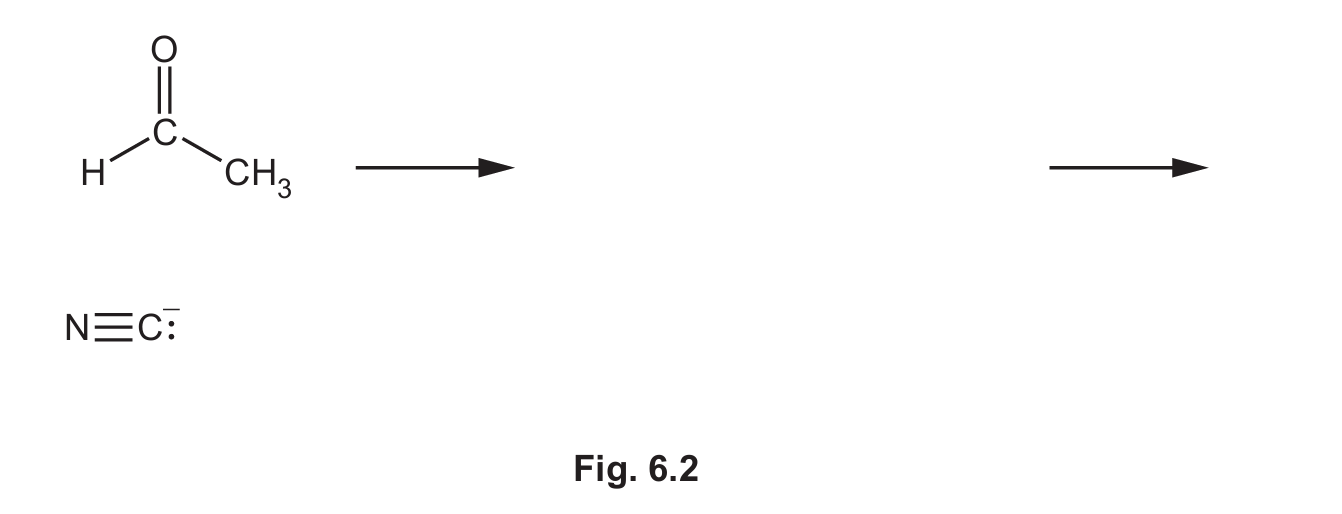
(ii) Complete Fig. 6.2 to show the mechanism for the reaction in step 1. Include all products, charges, dipoles, lone pairs of electrons and curly arrows, as appropriate.
(iii) Suggest a suitable reagent and conditions for step 2 in Fig. 6.1.
(iv) Fig. 6.3 shows the infrared spectrum of W, CH2=CHCN.
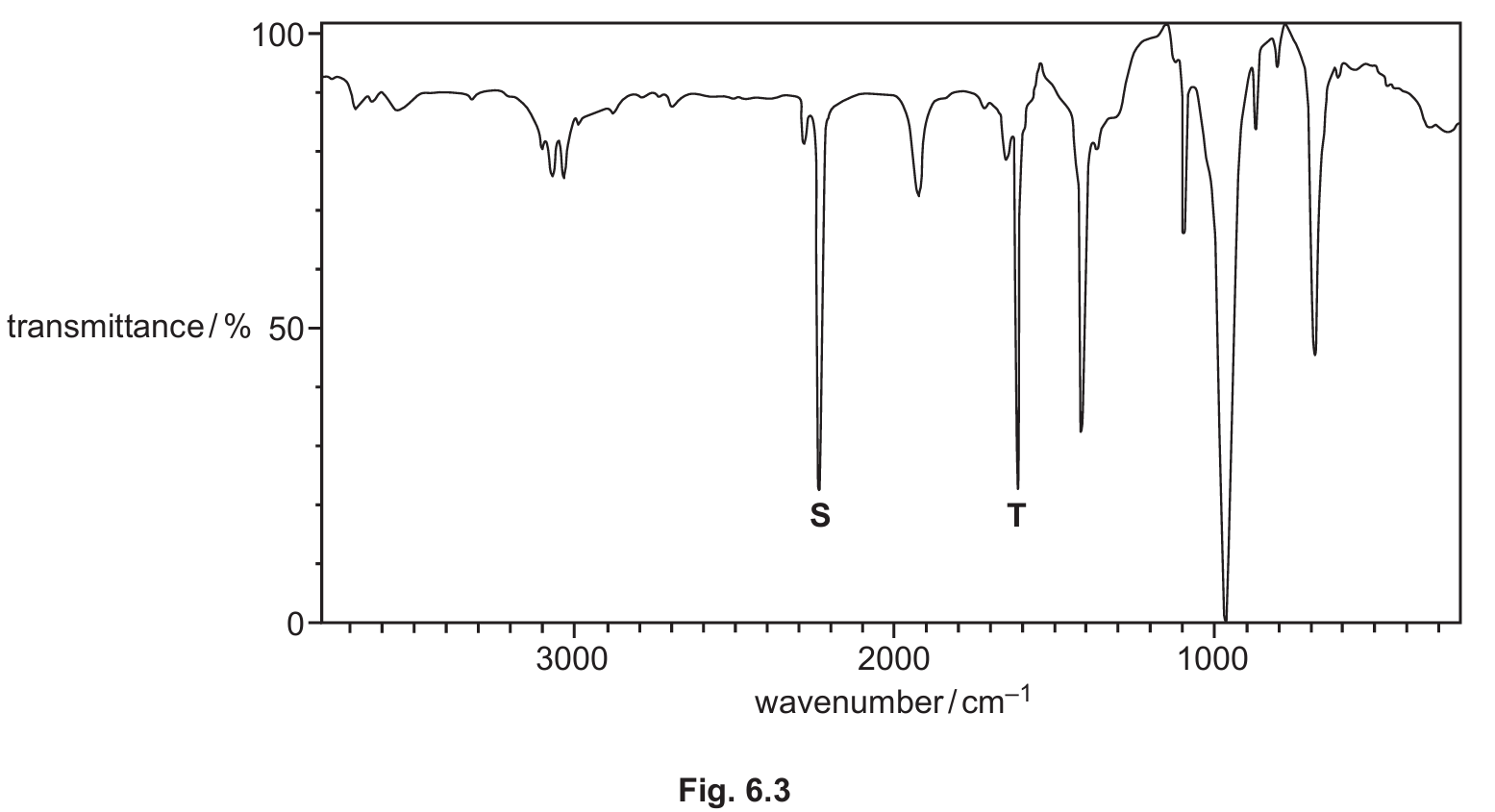
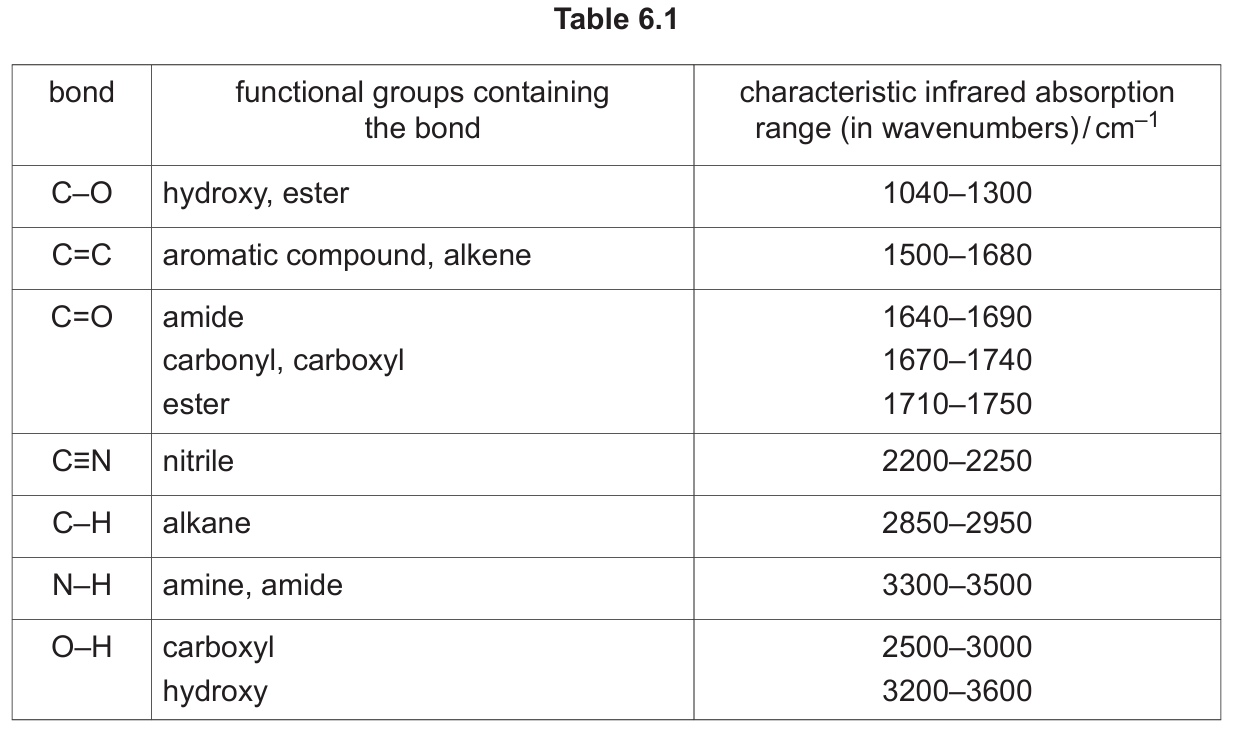
Use Table 6.1 to identify the bonds responsible for the absorptions marked S and T on Fig. 6.3.
(c) Molecules of W, CH2=CHCN, do not show stereoisomerism.
(i) Describe stereoisomerism.
(ii) Describe the two essential features of an alkene molecule that cause it to show geometrical stereoisomerism.
(d) Molecules of CH3CH(OH)CN exist as a pair of optical isomers.
Draw three-dimensional diagrams in the boxes to show the optical isomers of CH3CH(OH)CN.

(e) Propanenitrile is heated with hydrogen gas and a platinum catalyst. The only product is propylamine.
Construct an equation for this reaction.
(f) Propylamine can also be formed in a two-step synthesis from propan-1-ol, as shown in Fig. 6.4.
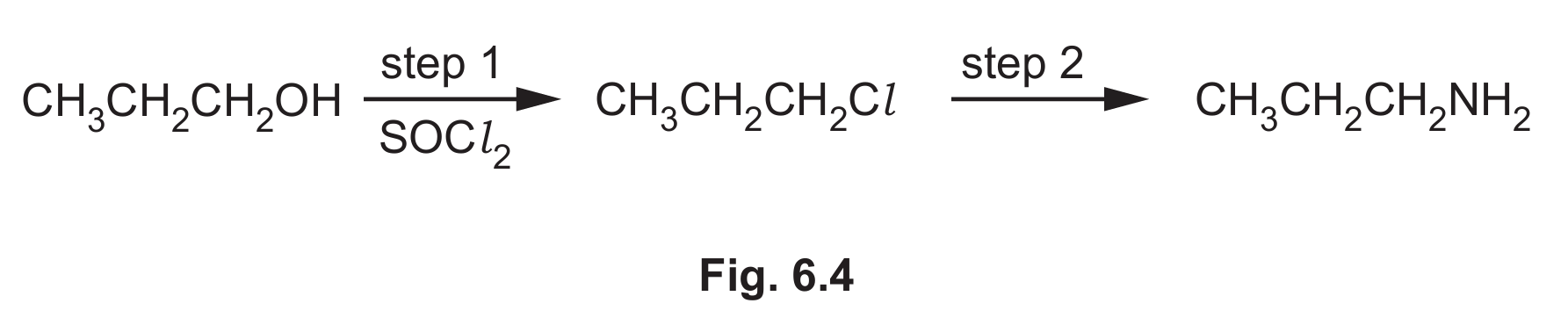
(i) Name the type of reaction in step 1 in Fig. 6.4.
(ii) Identify the reagent and conditions for step 2 in Fig. 6.4.
▶️ Answer/Explanation
(a)
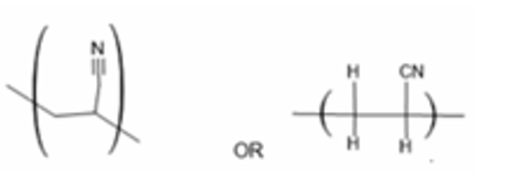
One repeat unit: \(\text{-}[\text{CH}_2\text{-CH}(\text{CN})]\text{-}\)
Explanation: The addition polymer of CH2=CHCN (acrylonitrile) is polyacrylonitrile (PAN). The double bond opens up, and the monomers add together. The repeat unit is derived from the monomer by replacing the double bond with a single bond and extending the bonds on either side.
(b)(i) Nucleophilic addition
Explanation: The reaction involves the nucleophile cyanide ion (:CN–) attacking the electrophilic carbon of the carbonyl group (C=O) in ethanal. This is the defining characteristic of a nucleophilic addition reaction.
(b)(ii)
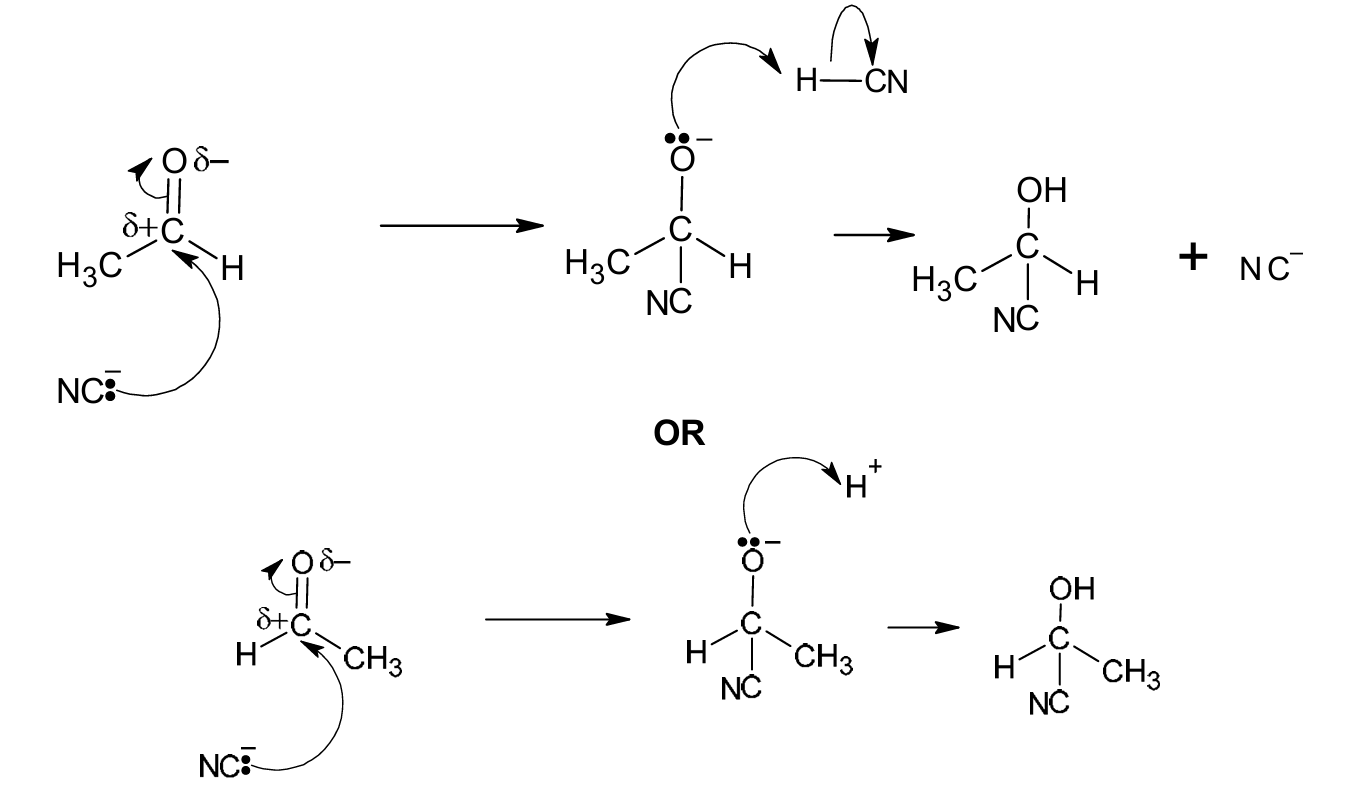
Explanation: The mechanism involves three key steps:
1. (M1) A curly arrow must show the lone pair from the carbon of the cyanide ion (the nucleophile) attacking the δ+ carbon atom of the polar carbonyl group. A dipole must be shown on the C=O bond (δ+ on C, δ- on O).
2. (M2) This forms a tetrahedral intermediate with a negative charge on the oxygen atom.
3. (M3) A curly arrow must show a lone pair from the negatively charged oxygen atom abstracting a proton (H+) from the environment (often represented as HCN or H3O+), resulting in the final product, 2-hydroxypropanenitrile (CH3CH(OH)CN).
(b)(iii) Reagent: Concentrated sulfuric acid OR concentrated phosphoric acid OR Al2O3 catalyst. Condition: Heat.
Explanation: Step 2 is a dehydration reaction, removing a molecule of water from the hydroxy-nitrile (CH3CH(OH)CN) to form the unsaturated nitrile (CH2=CHCN). Strong acids like H2SO4 or H3PO4 catalyze this elimination, as does heated aluminium oxide.
(b)(iv) S: C≡N AND T: C=C
Explanation: Referring to Table 6.1, the nitrile group (C≡N) has a characteristic strong, sharp absorption in the range 2200–2250 cm-1. The alkene group (C=C) has an absorption in the range 1500–1680 cm-1. These are the two key functional groups in the structure of W (CH2=CHCN).
(c)(i) Stereoisomerism is when molecules/compounds have the same structural formula (and same molecular formula) but a different arrangement of atoms/groups in space.
Explanation: This is the fundamental definition. Structural isomers have different atom connectivity, whereas stereoisomers have the same connectivity but differ in how their atoms are oriented in three-dimensional space.
(c)(ii)
1. (M1) There must be restricted rotation around the C=C double bond.
2. (M2) Each carbon atom involved in the double bond must have two different atoms or groups attached to it.
Explanation: The double bond’s pi bond prevents free rotation, locking the structure. If both carbons have two identical groups (e.g., in ethene, H2C=CH2), rotating the ends doesn’t create a distinct isomer. For geometrical isomerism (E/Z isomerism) to exist, each carbon of the double bond must have two different substituents, allowing for two distinct spatial arrangements (e.g., the groups can be on the same side, cis/Z, or opposite sides, trans/E).
(d)
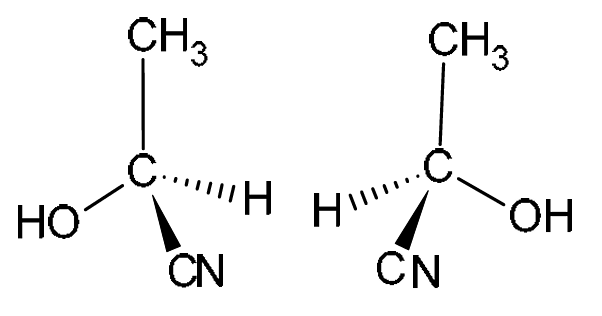
Explanation: CH3CH(OH)CN (2-hydroxypropanenitrile) has a chiral center (the carbon atom with the H, OH, CH3, and CN groups attached). This means it can exist as two non-superimposable mirror images. The 3D diagrams must show this, using wedges (coming out of the plane) and dashed lines (going into the plane) to represent the tetrahedral arrangement around the chiral carbon. The two isomers must be mirror images of each other.
(e) \(\text{CH}_3\text{CH}_2\text{CN} + 2\text{H}_2 \rightarrow \text{CH}_3\text{CH}_2\text{CH}_2\text{NH}_2\)
Explanation: This is a reduction reaction. The nitrile group (-C≡N) is reduced to a primary amine group (-CH2-NH2) using hydrogen gas and a metal catalyst like platinum (Pt), nickel (Ni), or palladium (Pd). Two moles of hydrogen are required for this conversion.
(f)(i) Substitution
Explanation: In step 1, the hydroxyl group (-OH) of propan-1-ol is replaced (substituted) by a chlorine atom from SOCl2 (thionyl chloride) to form chloro propane. This is a nucleophilic substitution reaction.
(f)(ii)
Reagent: Ammonia (NH3)
Conditions: Heat, and the reaction is often carried out in a sealed tube/under pressure or in ethanol as a solvent.
Explanation: Step 2 is another nucleophilic substitution reaction. The ammonia molecule (a nucleophile) attacks the chloroalkane, displacing the chloride ion and forming a primary amine (propylamine). Excess ammonia is often used to minimize further substitution (e.g., forming secondary and tertiary amines). Heating is required to overcome the activation energy for this substitution, and performing the reaction under pressure or in a sealed tube helps contain the reactants, especially since ammonia is a gas.
