Question
Compound F contains the elements carbon, hydrogen and oxygen only. All carbon-carbon bonds in F are single bonds. The structure of F was analysed by mass spectrometry and infra-red and NMR spectroscopy.
(a) The mass spectrum shows that the m/e value for the M peak is 90. The ratio of the heights of the M and M+1 peaks is 22.1:0.7.
(i) Use the ratio of the heights of the M and M+1 peaks to calculate the number of carbon atoms in a molecule of F.
(ii) Suggest the molecular formula of F.
molecular formula = C H O
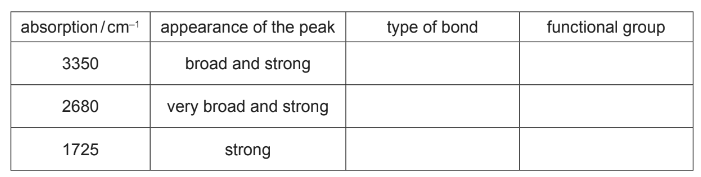
(b) The infra-red spectrum of F was obtained.
Use the Data Booklet and your knowledge of infra-red spectroscopy to identify the type of bond and the functional group responsible for these three absorptions.
(c) F was dissolved in deuterated trichloromethane, CDCl3, and the proton NMR spectrum of this solution obtained.
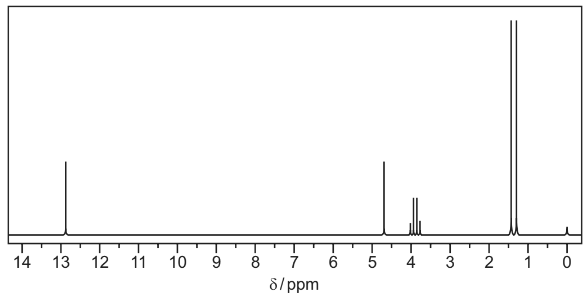
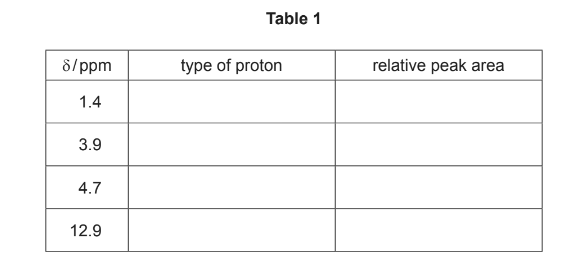
(i) Use the Data Booklet and your answer to (a)(ii) to complete Table 1 for the proton NMR
spectrum of F.
The actual chemical shifts for the four absorptions in F have been added for you.
(ii) Describe and explain the splitting pattern for the absorption at δ = 1.4.
(iii) F was dissolved in D2O and the proton NMR spectrum of this new solution obtained.
Two of the absorptions in Table 1 were not present in this spectrum.
Which absorptions were not present?
(iv) Suggest the structure of F.
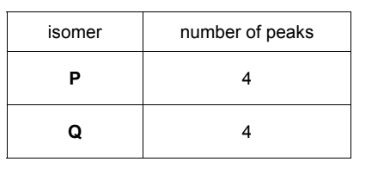
(d) Molecules of cycloheptadiene, C7H10, consist of a seven-membered ring with two
carbon-carbon double bonds.
(i) Complete the skeletal formulae of two isomers of cycloheptadiene.
The isomers P and Q were analysed using carbon-13 NMR spectroscopy.
(ii) Predict the number of peaks that will be seen in the carbon-13 NMR spectra of P and Q.

Answer/Explanation
Answer: (a)(i) 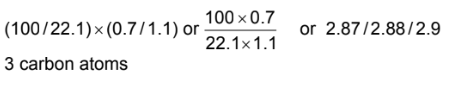
(a)(ii) C3H6O3
(b) 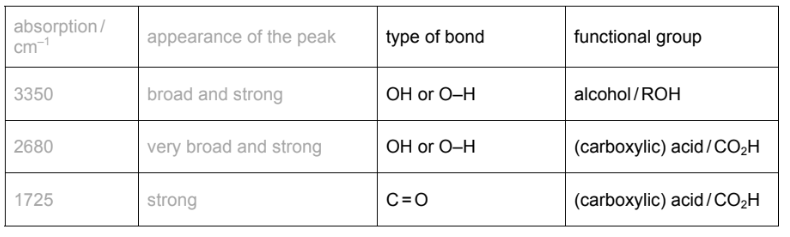
(c)(i) 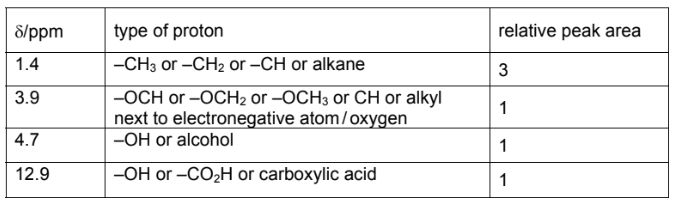
(c)(ii) doublet and 1 / one H/ proton on neighbouring OR adjacent carbon
(c)(iii) 4.7 and 12.9 OR –OH and –CO2H
(c)(iv) 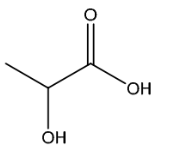
(d)(i) 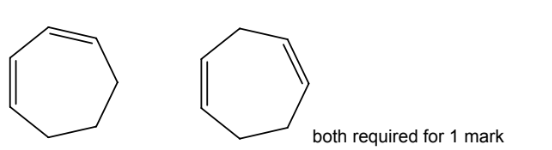
(d)(ii)