(a) V shows stereoisomerism.
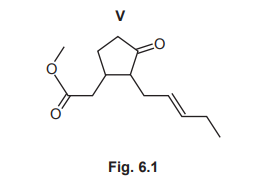
(i) Explain what is meant by stereoisomerism.
(ii) Deduce the number of stereoisomers of V. Explain your reasoning.
(iii) Deduce the molecular formula of V.
(iv) Name all the functional groups present in V.
(b) Fig. 6.2 shows two reactions involving V.
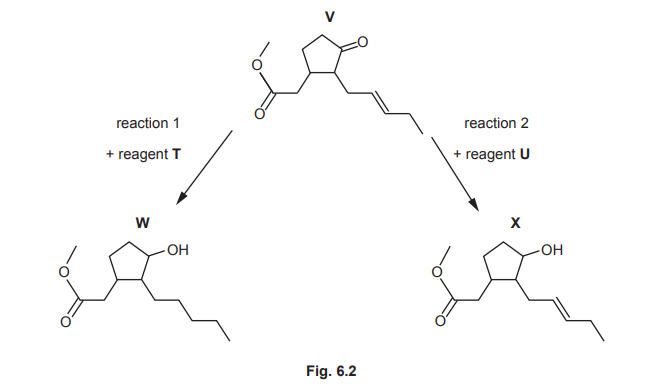
(i) Identify the role of reagent T for each functional group that reacts in reaction 1.
(ii) Suggest the identity of reagent U in reaction 2.
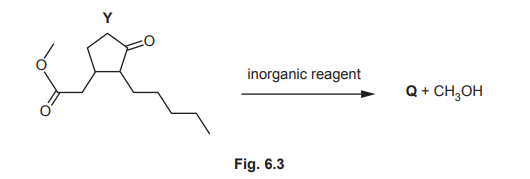
(c) Both functional groups in one molecule of Y react with an inorganic reagent to form one molecule of Q and one molecule of methanol, CH₃OH, as shown in Fig. 6.3.
(i) Part of the mass spectrum for Q is shown in Fig. 6.4. Only peaks with m/e greater than 198 are shown.
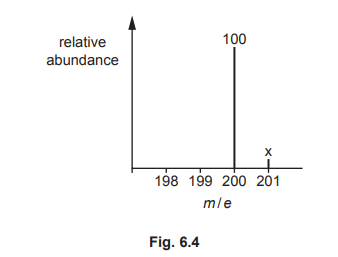
Calculate the relative abundance, x, of the peak at m/e = 201. Show your working.
(ii) Q contains only hydroxyl functional groups. Complete Table 6.1 to show the observations that occur when 2,4-dinitrophenylhydrazine (2,4-DNPH reagent) is added to separate samples of Y and Q.

(iii) Under certain conditions, 0.0020mol of Q reacts with an excess of sodium to produce a total of 44.8 cm³ of gas at s.t.p. Calculate the number of hydroxyl groups present in a molecule of Q. Show your working.
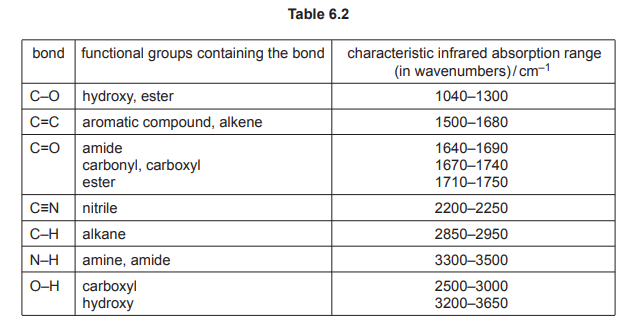
(iv) Use Table 6.2 to describe and explain two differences between the infrared spectrum of Y and Q in the region above 1500 cm⁻¹.
▶️ Answer/Explanation
(a)(i) Stereoisomerism refers to molecules with the same structural and molecular formula but different spatial arrangements of atoms/groups.
Explanation: Stereoisomers differ only in the 3D orientation of their atoms, such as in geometric (cis-trans) or optical isomers.
(a)(ii) Number of stereoisomers = 8.
Explanation: V has 2 chiral centers (each contributing 2 stereoisomers) and 1 C=C bond (contributing 2 geometric isomers), so total = \(2^3 = 8\).
(a)(iii) Molecular formula: \(C_{13}H_{20}O_3\).
Explanation: Counting all C, H, and O atoms in the structure gives the formula.
(a)(iv) Functional groups: ester, carbonyl (ketone), and C=C (alkene).
Explanation: The structure contains an ester (–COO–), a ketone (C=O), and a double bond (C=C).
(b)(i) Reagent T acts as a reducing agent for both C=O (carbonyl) and C=C (alkene) groups.
Explanation: T (likely \(H_2\)/Ni) reduces the ketone to an alcohol and the alkene to an alkane.
(b)(ii) Reagent U is sodium borohydride (\(NaBH_4\)).
Explanation: \(NaBH_4\) selectively reduces the carbonyl group without affecting the C=C bond.
(c)(i) Relative abundance \(x = 13.2\).
Explanation: From the mass spectrum, the ratio of peaks at m/e = 201 and 200 is 12:1.1. Thus, \(x = (12 \times 1.1) = 13.2\).
(c)(ii)
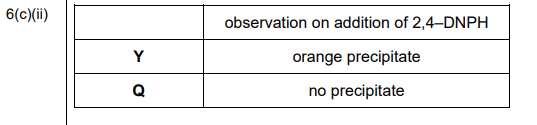
Explanation: Y (with C=O) forms an orange precipitate with 2,4-DNPH, while Q (no C=O) shows no reaction.
(c)(iii) Number of hydroxyl groups = 2.
Explanation: 0.002 mol Q produces 0.002 mol \(H_2\) (44.8 cm³ at STP). Since 2 OH groups produce 1 \(H_2\), Q must have 2 OH groups.
(c)(iv)
1. Y shows a C=O peak (1670–1740 cm⁻¹); Q does not.
2. Q shows an O-H peak (3200–3600 cm⁻¹); Y does not.
Explanation: Y has a ketone (C=O), while Q has alcohols (O-H) after reduction.
