(a) The equation for reaction 1 is shown.
reaction 1 \(X \to 2Y\)
Reaction 1 is first order with respect to the concentration of X. The half-life of the reaction, t1/2, is 900 s at 20°C.
(i) A solution of X with a concentration of 0.180 mol dm⁻³ is prepared at 20°C. Calculate the average rate of reaction 1 over the first 1800s.
(ii) Complete the rate equation for reaction 1.
(iii) Show that the rate constant, k, is \(7.70 × 10^{–4} s^{–1}\) at 20°C.
(iv) Calculate the initial rate of reaction 1 when the concentration of X is 0.150 mol dm⁻³. Include units.
(b) Catalysts may be homogeneous or heterogeneous.
(i) Platinum is a transition element. Explain why transition elements behave as catalysts.
(ii) Name the metal catalyst in the Haber process and explain why it is a heterogeneous catalyst.
(iii) Platinum acts as a heterogeneous catalyst in the removal of nitrogen dioxide, NO₂, from the exhaust gases of car engines. Describe the mode of action of a platinum catalyst in this process.
(iv) NO₂ acts as a homogeneous catalyst in the oxidation of atmospheric sulfur dioxide, SO₂. Write equations for the two reactions that occur.
(c) SO₂ dissolves in water, forming H₂SO₃. H₂SO₃ can be oxidised under acidic conditions. The relevant electrode reaction and its \(E^o\) value are shown.
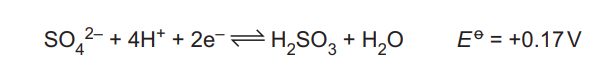
Four more half-equations for reactions occurring under acidic conditions, and their \(E^o\) values, are shown.
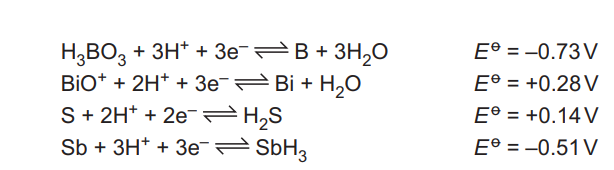
Select the oxidising agent that could oxidise H₂SO₃ to SO₄²⁻ ions under acidic conditions. Write an equation, and give the \(E^o\) cell value, for the reaction that occurs.
▶️ Answer/Explanation
(a)(i) Change in concentration = 0.135 mol dm⁻³ [1]
Average rate = 0.135 / 1800 = \(7.5 × 10^{–5}\) mol dm⁻³ s⁻¹
Explanation: For a first-order reaction, the concentration decreases exponentially. After two half-lives (1800 s), 75% of X remains unreacted. The change in concentration is \(0.180 – 0.045 = 0.135\) mol dm⁻³. Dividing by time gives the average rate.
(a)(ii) Rate = k[X]
Explanation: Since the reaction is first-order with respect to X, the rate equation is directly proportional to [X].
(a)(iii) k = 0.693 / t1/2 = 0.693 / 900 = \(7.70 × 10^{–4}\) s⁻¹
Explanation: The rate constant for a first-order reaction is derived from the half-life formula \(k = \frac{\ln 2}{t_{1/2}}\). Substituting the given half-life confirms the value of k.
(a)(iv) Initial rate = \(7.70 × 10^{–4} × 0.150 = 1.16 × 10^{–4}\) mol dm⁻³ s⁻¹
Explanation: Using the rate equation Rate = k[X], substituting k and [X] = 0.150 mol dm⁻³ gives the initial rate.
(b)(i) Any two from:
• Variable oxidation state
• Vacant/empty/unfilled d orbitals
• Can form dative bonds/accept electrons
Explanation: Transition elements exhibit catalytic activity due to their ability to adopt multiple oxidation states and interact with reactants via d-orbitals.
(b)(ii) Iron AND
Iron is solid, reactants are gases OR
Catalyst and reactants are in different phases/states.
Explanation: In the Haber process, iron is a heterogeneous catalyst because it is in a different phase (solid) than the gaseous reactants (N₂ and H₂).
(b)(iii) Two for one mark, three for two marks:
• Adsorption of reactants by Pt
• Bonds of reactants weaken
• Desorption of products
Explanation: Platinum facilitates the reaction by adsorbing NO₂, weakening its bonds, and releasing products like N₂ and O₂.
(b)(iv) \(NO_2 + SO_2 \to NO + SO_3\) AND
\(2NO + O_2 \to 2NO_2\)
Explanation: NO₂ catalyzes SO₂ oxidation by first oxidizing SO₂ to SO₃ and then being regenerated by reaction with O₂.
(c) \(BiO^+\)
3H₂SO₃ + 2BiO⁺ + H₂O → 3SO₄²⁻ + 8H⁺ + 2Bi
\(E^o\) cell = 0.11 V
Explanation: \(BiO^+\) is the strongest oxidising agent (\(E^o = +0.32\) V) that can oxidize H₂SO₃ (\(E^o = +0.21\) V). The cell potential is \(0.32 – 0.21 = 0.11\) V.
