(a) Predict and explain the variation in the enthalpy change of hydration for the ions \(F^–\), \(Cl^–\), \(Br^–\), and \(I^–\).
(b) Fig. 2.1 shows an incomplete energy cycle involving calcium fluoride, CaF₂.
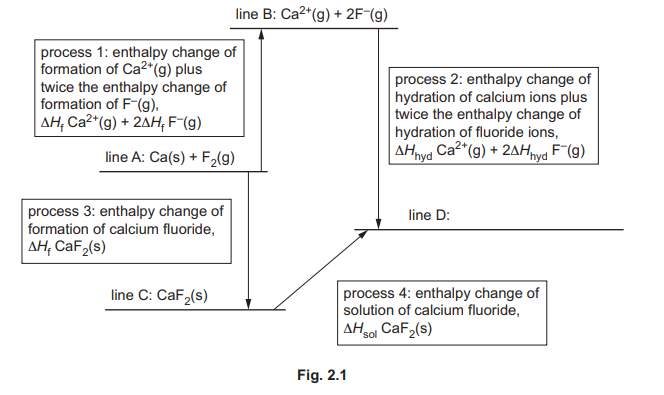
(i) Complete line D. Include state symbols.
(ii) Identify the five enthalpy changes needed to calculate process 1.
(iii) Define lattice energy, \(\Delta H_{latt}\).
(iv) Relate \(\Delta H_{latt}\) to processes 1 and 3.
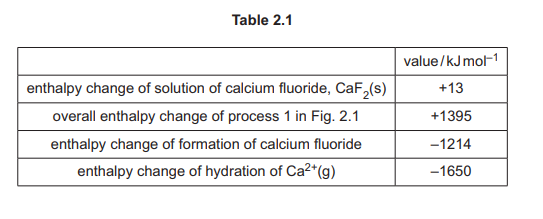
(c) Calculate \(\Delta H_{hyd}\) of \(F^–(g)\) using Table 2.1.
(d) Define entropy.
(e) Calculate ΔS for the solution of compound T at 298K.
(f) Predict solubility change of T when heated from 298K to 360K.
▶️ Answer/Explanation
(a) The enthalpy change of hydration becomes less exothermic from \(F^–\) to \(I^–\). This occurs because larger ions (increasing ionic radius) have weaker ion-dipole interactions with water molecules.
(b)(i) Line D represents the aqueous state: \(CaF_2(aq)\) or \(Ca^{2+}(aq) + 2F^-(aq)\).
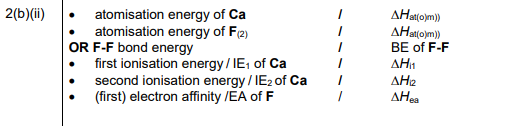
(b)(ii) The five required enthalpy changes are:
1. Enthalpy of atomization of Ca (\(\Delta H_{at}(Ca)\))
2. Enthalpy of atomization of F₂ (\(\Delta H_{at}(F_2)\))
3. First ionization energy of Ca (\(IE_1(Ca)\))
4. Second ionization energy of Ca (\(IE_2(Ca)\))
5. Electron affinity of F (\(\Delta H_{ea}(F)\))
(b)(iii) Lattice energy (\(\Delta H_{latt}\)) is the energy released when one mole of ionic solid forms from its gaseous ions.
(b)(iv) \(\Delta H_{latt} = \Delta H_3 – \Delta H_1\)
(c) Using Hess’s Law with Table 2.1 data:
\(1395 – 1650 + 2\Delta H_{hyd}(F^-) = -1214 + 13\)
Solving gives \(\Delta H_{hyd}(F^-) = -473 \text{ kJ mol}^{-1}\)
(d) Entropy (ΔS) measures the number of possible microscopic arrangements of a system.
(e) Using \(\Delta G = \Delta H – T\Delta S\):
\(6.00 = 30.0 – 298\Delta S\)
\(\Delta S = 80.5 \text{ J mol}^{-1} K^{-1}\)
(f) Compound T becomes more soluble as temperature increases because ΔS is positive. The \(-T\Delta S\) term becomes more negative, making ΔG more negative.
