(a) A and B react together to give product AB.
\(A + B \to AB\)
When the concentrations of A and B are both 0.0100 moldm⁻³, the rate of formation of AB is 7.62 × 10⁻⁴ moldm⁻³ s⁻¹. When the concentrations of A and B are both 0.0200 moldm⁻³,
the rate of formation of AB is 3.05 × 10⁻³ moldm⁻³ s⁻¹.
(i) Complete the three possible rate equations that are consistent with these data.
(ii) Choose one of the rate equations you have written in (i), and calculate the value of the rate constant, k. Include the units of k.
(iii) Explain why it is not possible to calculate a value for the half-life, \(t_{\frac{1}{2}}\), of this reaction using the value of the rate constant k calculated in (ii) and the equation k = 0.693/\(t_{\frac{1}{2}}\).
(b) Catalysts may be homogeneous or heterogeneous.
(i) Identify two metals that act as heterogeneous catalysts in the removal of \(NO_2\) from the exhaust gases of car engines.
(ii) Iron acts as a heterogeneous catalyst in the Haber process. Describe the mode of action of this iron catalyst.
(iii) Fe²⁺ ions act as a homogeneous catalyst in the reaction between I⁻(aq) and S₂O₈²⁻(aq). Write equations for the two reactions that occur when Fe²⁺(aq) is added to a mixture of I⁻(aq) and S₂O₈²⁻(aq).
(iv) Explain the difference between a homogeneous catalyst and a heterogeneous catalyst.
(c) \(Fe^{2+}\) ions can be oxidised to \(Fe^{3+}\) ions under alkaline conditions by suitable oxidising agents.
(i) Iron is a transition element. Explain why iron forms stable compounds in both the +2 and the +3 oxidation states.
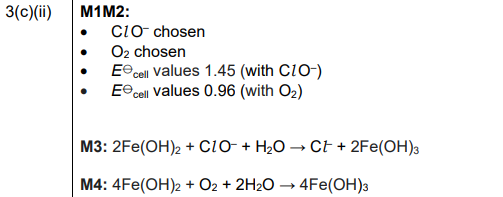
(ii) The half-equation for the reduction of Fe³⁺ under alkaline conditions, and its \(E^o\) value, are shown.
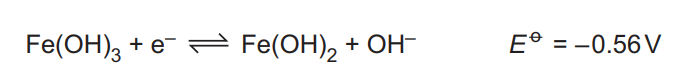
Four more half-equations for reactions under alkaline conditions, and their \(E^o\) values, are shown. 
Select two oxidising agents that can oxidise Fe²⁺ ions to Fe³⁺ ions under alkaline conditions. Write an equation, and give the \(E^0_{cell}\) value, for each of the two reactions that occur
▶️ Answer/Explanation
(a)(i) Three possible rate equations:
1. rate = k[A][B]
2. rate = k[A]²
3. rate = k[B]²
Explanation: When both concentrations double, the rate quadruples (from 7.62×10⁻⁴ to 3.05×10⁻³), indicating a second-order reaction. The rate could depend on either [A]², [B]², or [A][B].
(a)(ii) Calculation of rate constant k:
Using rate = k[A][B]:
k = rate / ([A][B]) = 7.62 × 10⁻⁴ / (0.01 × 0.01) = 7.62 mol⁻¹ dm³ s⁻¹
Explanation: The units of k are derived from the second-order rate equation (mol⁻¹ dm³ s⁻¹).
(a)(iii) Half-life explanation:
The equation \(k = 0.693/t_{1/2}\) applies only to first-order reactions. Since this reaction is second-order, the half-life depends on initial concentrations and cannot be calculated using this formula.
(b)(i) Heterogeneous catalysts: Platinum (Pt) and Rhodium (Rh).
Explanation: These metals are used in catalytic converters to reduce NO₂ emissions from car exhausts.
(b)(ii) Mode of action of iron catalyst:
1. Adsorption of N₂ and H₂ onto the iron surface.
2. Weakening of N≡N and H-H bonds.
3. Formation of NH₃, which then desorbs from the surface.
Explanation: The iron catalyst provides a surface for reactant molecules to interact, lowering the activation energy.
(b)(iii) Homogeneous catalysis equations:
1. \(S_2O_8^{2-} + 2Fe^{2+} → 2SO_4^{2-} + 2Fe^{3+}\)
2. \(2Fe^{3+} + 2I^- → 2Fe^{2+} + I_2\)
Explanation: Fe²⁺ is oxidised to Fe³⁺ by S₂O₈²⁻, then Fe³⁺ oxidises I⁻ to I₂ while being reduced back to Fe²⁺.
(b)(iv) Catalyst difference:
• Homogeneous: Catalyst and reactants in the same phase (e.g., all in solution).
• Heterogeneous: Catalyst and reactants in different phases (e.g., solid catalyst with gaseous reactants).
(c)(i) Stable oxidation states:
Iron exhibits stable +2 and +3 states because its 3d and 4s orbitals have similar energies, allowing variable electron loss.
(c)(ii) Selected oxidising agents:
1. \(H_2O_2 + 2Fe^{2+} → 2Fe^{3+} + 2OH^-\) (E°cell = +1.71 V)
2. \(ClO^- + 2Fe^{2+} + H_2O → 2Fe^{3+} + Cl^- + 2OH^-\) (E°cell = +1.70 V)
Explanation: Both reactions have positive E°cell values, indicating spontaneous oxidation of Fe²⁺ under alkaline conditions.